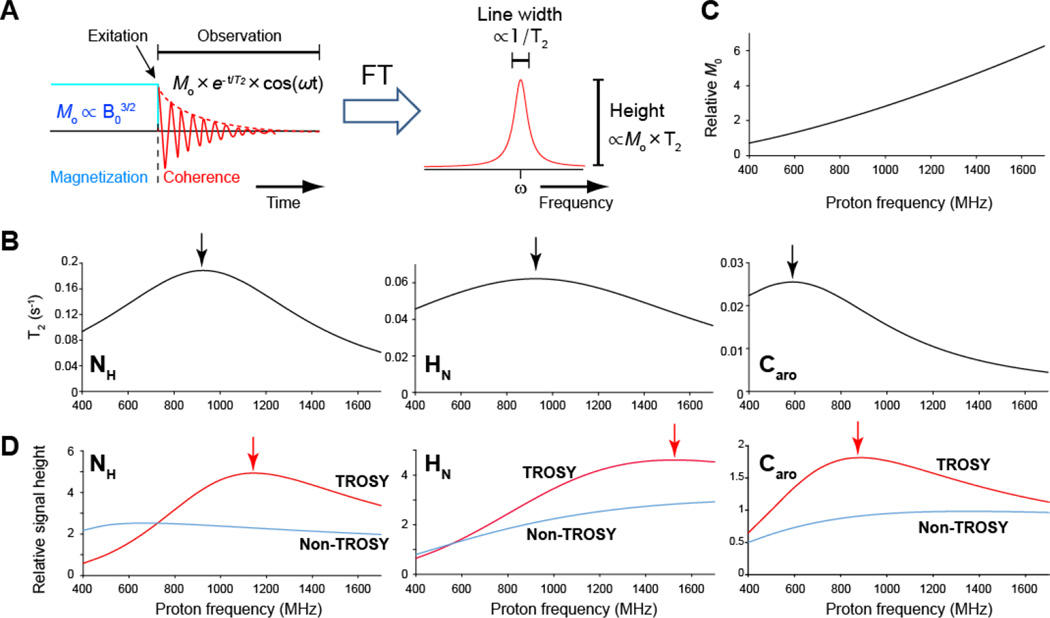
Figure 1. Resolution and sensitivity of TROSY resonances.
(A) Relation of T2 and initial magnetization (M0) in time domain to resolution (line width) and sensitivity (signal height), respectively, in frequency domain. (B) T2 of the 15NH, 1HN, and 13Caro TROSY resonances for the protein with rotational correlation time of 20 ns. For the 15NH and 1HN TROSY resonances, α-helical part of 2H15N labeled protein is assumed. For the 13Caro TROSY resonances, non-deuterated altenately13C-12C labeled protein that is labeled with 3-13C labeled pyruvate was assumed. Detail of the calculation of T2 are shown below. (C) Field dependence of M0 relative to that at 500 MHz. (D) 1D Signal height of TROSY (red) and conventional decoupled non-TROSY (cyan) resonances relative to the signal height of TROSY resonances at 500 MHz. The signal height for the non-TROSY resonances are doubled.