Summary
Satellite cells, the predominant stem cell population in adult skeletal muscle, are activated in response to hypertrophic stimuli and give rise to myogenic progenitor cells (MPCs) within the extracellular matrix (ECM) that surrounds myofibers. This ECM is composed largely of collagens secreted by interstitial fibrogenic cells which influence satellite cell activity and muscle repair during hypertrophy and aging. Here, we show MPCs interact with interstitial fibrogenic cells to ensure proper ECM deposition and optimal muscle remodeling in response to hypertrophic stimuli. MPC-dependent ECM remodeling during the first week of a growth stimulus is sufficient to ensure long-term myofiber hypertrophy. MPCs secrete exosomes containing miR-206 which represses Rrbp1, a master regulator of collagen biosynthesis, in fibrogenic cells to prevent excessive ECM deposition. These findings provide insights into how skeletal stem and progenitor cells interact with other cell types to actively regulate their extracellular environments for tissue maintenance and adaptation.
Keywords: Muscle extracellular matrix, satellite cells, Pax7, Dicer, miRNA
Graphical abstract
eTOC Blurb
Stem cells interact with the surrounding extracellular environment to facilitate tissue plasticity. Fry and colleagues report that skeletal muscle myogenic progenitor cells (MPCs) secrete exosomes containing miR-206 which regulates fibrogenic cell collagen expression through repression of Rrbp1. MPC-mediated regulation of the muscle extracellular environment is necessary during early myofiber growth.
Introduction
During growth and repair, interactions between muscle stem cells, known as satellite cells, and the extracellular matrix (ECM) in skeletal muscle are crucial for muscle adaptation Satellite cells reside between the sarcolemma and basal lamina of muscle fibers (Mauro, 1961), and upon activation, either self-renew or proliferate to give rise to myogenic progenitor cells that are able to migrate into the ECM. Muscle ECM is largely comprised of various collagen isoforms, secreted primarily by muscle fibroblasts present in the interstitial space between fibers (Sanderson et al., 1986; Zou et al., 2008). Several groups have shown that the interstitial muscle niche facilitates activation of satellite cells following exposure to external stimuli associated with muscle growth and repair (Barberi et al., 2013; Conboy and Rando, 2002; Le Grand et al., 2009; Shea et al., 2010; Urciuolo et al., 2013). Specifically, the composition and mechanical properties of the ECM regulate satellite cell activity and renewal (Calve et al., 2010; Gilbert et al., 2010b). While evidence exists for muscle-mediated regulation of ECM production (Braghetta et al., 2008), the role of activated satellite cells, and their daughters, myogenic progenitor cells (MPCs), in the modulation of their extracellular niche during muscle adaptation is largely unexplored.
Genetic depletion of satellite cells results in an increase in muscle ECM during mechanical overload-induced hypertrophy (Fry et al., 2014), repair (Murphy et al., 2011) and aging (Fry et al., 2015; Lee et al., 2015). Using the tamoxifen-inducible Pax7-DTA mouse, we previously demonstrated that following 8-weeks of mechanical overload of satellite cell-depleted muscle, hypertrophy was attenuated, accompanied by a substantial increase in ECM content (Fry et al., 2014). Consistent with the significant increase in ECM content, a wide array of collagen genes were overexpressed in satellite cell-depleted muscle undergoing hypertrophy. Increased production of collagens during growth and repair in muscle serves to provide scaffolding for nascent muscle fibers and satellite cells (Kaariainen et al., 2000; Kjaer et al., 2006); however, unchecked accumulation of muscle ECM impedes muscle repair and mechanical function (Sato et al., 2003). Thus, the precise regulation of muscle ECM production is necessary during periods of adaptation to insure optimal muscle function.
Our in vitro studies show that MPCs actively secrete factors that regulate muscle fibroblast ECM gene expression that appear to be independent of the TGF-β pathway (Fry et al., 2014). Intercellular communication is mediated through a number of different mechanisms, with extracellular vesicles such as exosomes emerging as important players in cell-to-cell communication (Kourembanas, 2015). Exosomes contain host cell-derived RNA and protein and have been shown to be capable of transferring both mRNA and miRNAs to target cells (Hergenreider et al., 2012; Valadi et al., 2007). Thus, the secretion of exosomes by MPCs provides a heretofore unrecognized mechanism for regulating the ECM production of fibrogenic cells during muscle remodeling. The purpose of this study was to delineate the underlying mechanism by which MPCs regulate their extracellular environment during hypertrophy, thereby identifying a role of activated satellite cells in skeletal muscle remodeling.
Results
Satellite cell depletion does not affect fibrogenic cell abundance during the early phases of hypertrophy
The excessive accumulation of ECM following 8 weeks of mechanical overload in satellite cell-depleted muscle is associated with increased abundance of Tcf4+ fibrogenic cells isolated from muscle; however, in vitro co-culture of Tcf4+ cells with MPCs did not affect their proliferation (Fry et al., 2014). To determine if increased collagen gene expression observed early during overload is due to increased fibrogenic cell content (Fry et al., 2014), muscle was analyzed following one and two weeks of mechanical overload in response to synergist ablation (SA) surgery, as outlined in Figure 1A. Tamoxifen treatment resulted in greater than 90% satellite cell depletion (SC-Dep) compared to vehicle (SC-WT) which did not affect growth at 1 week (SA1) or 2 weeks (SA2) (Figure S1), consistent with our previous work (Fry et al., 2014; McCarthy et al., 2011). As shown in Figure 1, Tcf4+ cell abundance increased in response to overload in both satellite cell-depleted and wild type muscle (representative image, Figures 1B quantified in Figure 1F). No difference in Tcf4+ cell number nor myofibroblast differentiation was apparent, the latter identified by α smooth muscle actin (αSMA) expression (representative image Figure 1C, overlayed with Tcf4 staining in Figure 1E). Only a small percentage of Tcf4+ cells were also αSMA+ (Figure 1G). By 8 weeks, the number of fibrogenic cells was declined, but remained elevated in satellite cell-depleted muscle compared to vehicle controls (Figure 1H). Thus, as suggested from in vitro data (Fry et al., 2014), MPCs appear to interfere with fibrogenic cell collagen gene expression early during hypertrophy so that in the absence of MPCs, fibrogenic cell collagen gene expression increases, with no significant effect on fibrogenic cell number or state of differentiation.
Figure 1. Depletion of satellite cells does not influence fibrogenic cell expansion or myofibroblast differentiation during the first two weeks of overload.
A) Experimental schematic for conditional depletion of satellite cells using the Pax7-DTA mouse strain. Following tamoxifen or vehicle treatment and a two week washout, plantaris muscle was mechanically overloaded using synergist ablation for either one (SA1) or two (SA2) weeks. See also Figure S1 and S2A–B. Representative images of a SC-Dep muscle cross-section at SA1 illustrating immunohistochemical identification of (B) Tcf4+ (green); (C) α smooth muscle actin (αSMA) + (orange) cells. Blood vessels (white arrows) are strongly αSMA+, serving as a positive control. D) DAPI staining of nuclei. E) Merged image of Tcf4, αSMA and DAPI staining in the SC-Dep muscle cross-section. The orange arrowhead in B-E identifies a rare Tcf4+/αSMA+ myofibroblast. Scale bar=20 µM. F) Quantification of Tcf4+ fibrogenic cells in SC-WT and SC-Dep skeletal muscle at baseline (sham), SA1 and SA2. † denotes significant difference from sham; p <0.05 (N = 5 mice/group). G) Quantification of percentage of Tcf4+ cells that also express αSMA in SC-WT and SC-Dep skeletal muscle at baseline (sham), SA1 and SA2. † denotes significant difference from sham; p <0.05 (N = 5 mice/group). H) Quantification of fold change in Tcf4+ fibrogenic cells in SC-WT and SC-Dep skeletal muscle at baseline (sham) and SA8. † denotes significant difference from sham; ‡ denotes significant difference from SC-WT; p <0.05 (N = 6 mice/group). All data presented as Mean ± SEM.
microRNAs present in MPC exosomes down-regulate fibrogenic cell collagen expression
We previously demonstrated increased muscle collagen content in satellite cell-depleted mice and a negative effect of MPCs on muscle fibrogenic cell collagen gene expression in vitro (Fry et al., 2014). In that study, elevated collagen gene expression was not associated with increased SMAD phosphorylation, suggesting that TGF-β signaling, known to promote muscle fibrosis, was not responsible. Similarly, in the current study, pSMAD+ nuclei were not increased in satellite cell-depleted compared to wild type muscle; however, pSMAD3+ nuclei did increase in both groups following 1 week of overload (representative image Figure S2A, quantified in Figure S2B). We hypothesized that MPCs communicate with fibrogenic cells through an exosome-mediated mechanism. To test this hypothesis, exosomes were isolated from MPC conditioned media (CM), which was confirmed using western blot analyses of proteins enriched in exosomal membranes compared to fibrogenic cells and MPCs (Figure S2C). Figure 2A shows that MPC CM and MPC CM-derived exosomes down-regulate the expression of mRNAs encoding the three most abundant collagens in muscle and fibronectin in primary skeletal muscle fibrogenic cells. Whereas fibrogenic cell CM had no effect on collagen gene expression, exosomes isolated from MPC CM and added to fibrogenic cell CM resulted in the down regulation of collagen 1, 3 and 6 and fibronectin gene expression. These data extend our previous observations that MPCs participate in paracrine-mediated regulation of fibrogenic cell collagen expression (Fry et al., 2014). Delivery of MPC exosome content to fibrogenic cells was demonstrated by labeling exosomal RNA with acridine orange, and then culturing labeled MPC exosomes with fibrogenic cells. The appearance of labeled RNA within fibrogenic cells began 2–4 hr after culture with labeled MPC exosomes, demonstrating the docking of MPC exosomes and delivery of their contents to fibrogenic cells (Figure 2B). Exosomes represent a relatively new mechanism of cellular communication, and exosomes from donor cells have been shown to initiate changes in the phenotype of target cells (Bang et al., 2014), including fibroblasts (Webber et al., 2010).
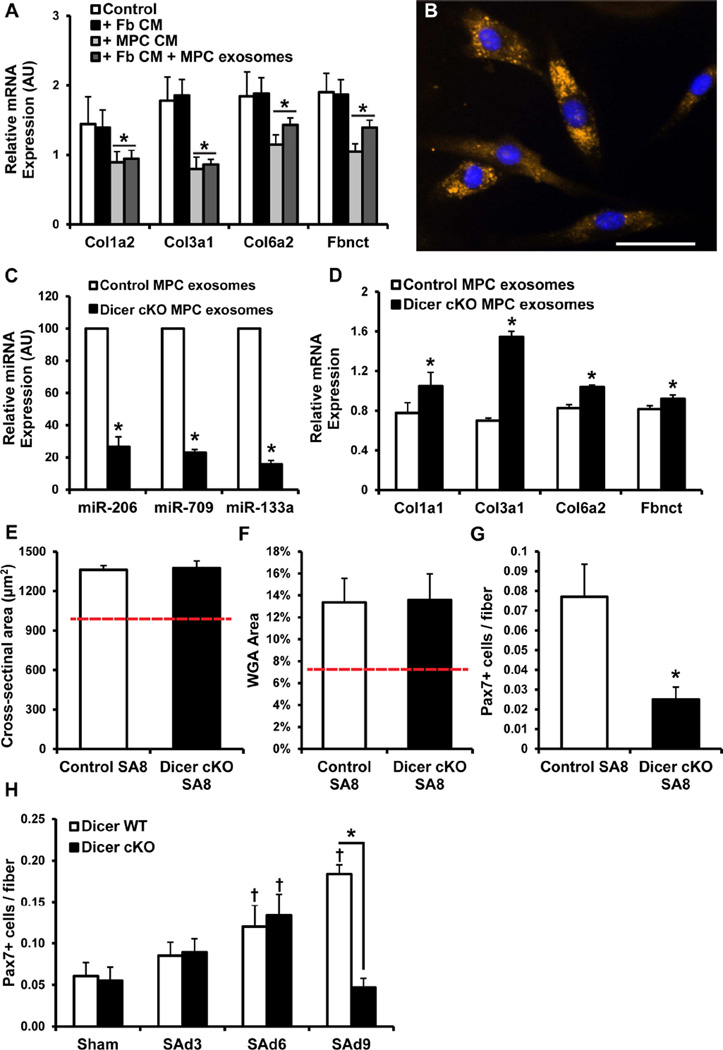
Figure 2. Dicer deletion affects myogenic progenitor cell (MPC) exosome-mediated regulation of fibrogenic cell ECM mRNA accumulation in vitro and MPC viability in vivo.
A) Primary fibrogenic cell collagen and fibronectin mRNA expression following culture for 24 hr in control medium, fibrogenic (Fb) conditioned medium (CM), MPC CM, and Fb CM + MPC exosomes. See also Figure S2C and S6 and Table S1–S3. * denotes significant effect of MPC CM/MPC exosomes; p < 0.05 (N = 3–5 isolates studied in duplicate). B) Primary fibrogenic cells containing acridine orange-labeled RNA from MPC exosomes. Scale bar = 50 µm. C) Select Pax7-Dicer MPC exosome miRNA expression levels following vehicle (Control) or tamoxifen treatment to conditionally knock out (cKO) dicer activity. * denotes significant effect of Dicer cKO; p < 0.05 (N = 2 isolates studied in duplicate). D) Primary fibrogenic cell collagen mRNA expression. * denotes significant effect of Dicer cKO; p < 0.05 (N = 3 isolates studied in duplicate). Quantification of E) average cross-sectional area, F) α-wheat germ agglutinin (WGA) stained area as a percent of total muscle areas, and G) Pax7+ satellite cells in the plantaris from Dicer cKO and Control SA8 muscle. Dashed red line in E and F indicates sham values. * denotes significant difference between Dicer cKO and Control; p < 0.05 (N = 4 animals/group). H) Quantification of satellite cell content in Dicer cKO and Control skeletal muscle during days 3 (SAd3), 6 (SAd6) and 9 (SAd9) of overload. * denotes significant difference between WT and cKO within the respective group; † significant increase relative to sham condition within that respective time point; p < 0.05 (N = 3 animals/group/time point). All data presented as Mean ± SEM.
Exosomes have been demonstrated to contain miRNAs (Bang et al., 2014), which negatively regulate target mRNA stability and/or translation (Pillai et al., 2007; Valencia-Sanchez et al., 2006). Additionally, miRNAs contained in exosomes can explicitly down-regulate target cell mRNA expression (Umezu et al., 2014). To test the hypothesis that miRNAs packaged in exosomes are responsible for MPC-mediated regulation of fibrogenic cell collagen production, primary MPCs were isolated from adult skeletal muscle of the Pax7CreER/+;Dicerfl/fl mouse, designated as Pax7-Dicer. The Pax7-Dicer strain allows for the specific and conditional depletion of miRNAs in satellite cells. Treatment with tamoxifen activates Cre recombinase, driven by the Pax7 promoter, only in satellite cells, which induces deletion of the RNase III domain of the Dicer gene, effectively eliminating Dicer-mediated processing of precursor miRNAs in satellite cells. Following in vitro treatment of MPCs from Pax7-Dicer mice with either 4-OH tamoxifen or vehicle (Dicer cKO vs control, respectively, Figures 2C and D), exosomes were isolated and miRNA expression, as well as Dicer mRNA, quantified. Exosomes isolated from Dicer cKO MPCs showed efficient Dicer knockdown (93%) and a 75–85% depletion of miRNA levels based on miR-206, -709 and -133a expression as compared to vehicle-treated control MPC exosomes (Figure 2C). Importantly, similar levels of miRNA depletion in satellite cells have been reported to have a physiological effect (Cheung et al., 2012). Incubation of muscle fibrogenic cells with exosomes isolated from control MPCs resulted in the down-regulation of fibrogenic cell collagen mRNA expression compared to those in which miRNAs were depleted (Dicer cKO; Figure 2D). Exosomes contain a variety of molecules (Gross et al., 2012; Valadi et al., 2007), and the rescue of fibrogenic cell collagen mRNA expression following incubation with miRNA-depleted exosomes provides strong evidence that miRNAs within exosomes are responsible for MPC-mediated inhibition of fibrogenic cell collagen production.
From the above in vitro results, we predicted that the tamoxifen-treated Pax7-cKO Dicer mouse would phenocopy the Pax7-DTA mouse, demonstrating impaired long term growth and increased ECM accumulation. On the contrary, following 8 weeks of overload, myofiber CSA (Figure 2E) and ECM accumulation (Figure 2F) were similar in Dicer cKO compared to control muscles. This was unexpected given that satellite cells were significantly depleted following 8 weeks of overload in the absence of Dicer activity (Figure 2G). However, during the first week of overload, satellite cell abundance increased through day 6 (SAd6, Figure 2H), but then demonstrated a significant decline by day 9 (SAd9, Figure 2H). This is consistent with the work of the Rando laboratory showing the necessity of Dicer for proper satellite cell maintenance (Cheung et al., 2012) and suggests that activated satellite cells/MPCs are particularly important in regulating the ECM early during the growth response, which we next directly tested (see Figure 5).
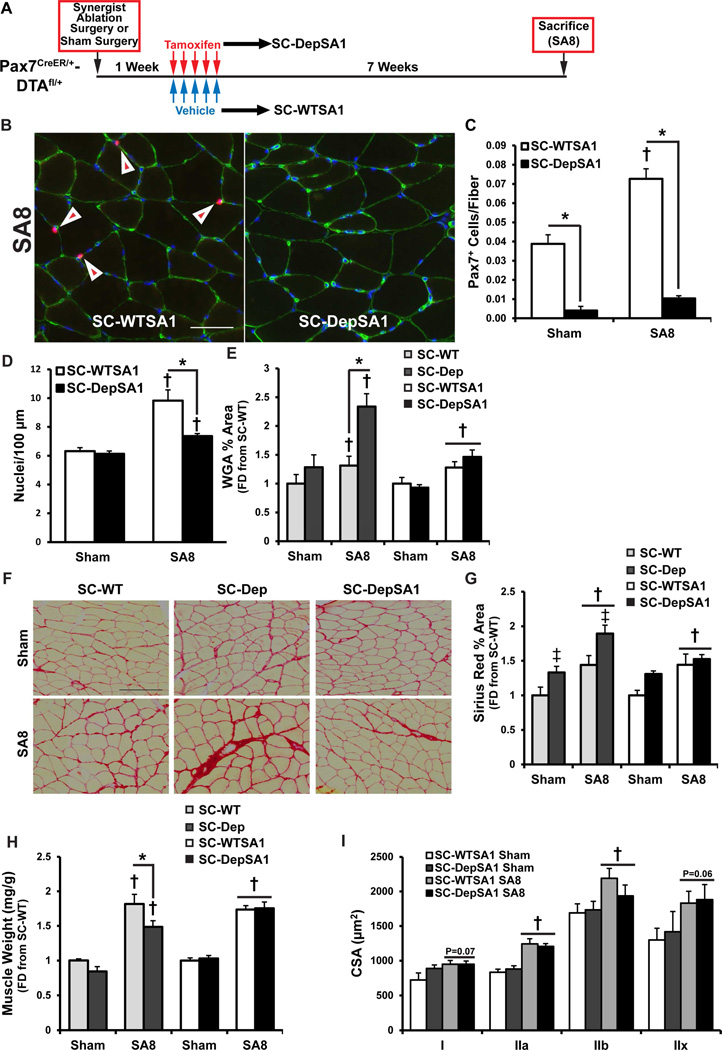
Figure 5. Presence of activated satellite cells/MPCs during the acute phase of ECM remodeling prevents excessive ECM accumulation and promotes long term growth.
A) Experimental schematic for conditional depletion of activated satellite cells following one week of mechanical overload (SC-DepSA1). Animals were mechanically overloaded using synergist ablation or sham operated, and then treated with tamoxifen or vehicle at SA1; sham or overload continued for an additional 7 weeks (Sham and SA8, respectively). B) Immunohistochemical identification of Pax7+ satellite cells (red) and laminin (green) in both SC-WTSA1 and SC-DepSA1 muscle cross-sections at SA8. Scale bar=50 µM. Arrows identify Pax7+ cells, quantified in C). * denotes significant difference between SC-WTSA1 and SC-DepSA1 within the respective time point; † significant increase relative to Sham condition within that respective group; p < 0.05 (N = 6–9 animals/group/time point). D) Quantification of the number of myonuclei in isolated fibers per 100 µM fiber length in SC-WTSA1 and SC-DepSA1 animals. * denotes significant difference between SC-WTSA1 and SC-DepSA1 within the respective time point; denotes † significant increase relative to Sham condition within that respective group; p < 0.05 (N = 5–8 animals/group/time point with data points from each animal generated from 15–20 fibers). E) Quantification of α-wheat germ agglutinin (WGA) area as a percent of total muscle area. Data are presented as the fold-difference from the Vehicle Sham condition within each experimental paradigm. * denotes significant difference between SC-WT and SC-Dep within the respective time point; † denotes significant increase relative to Sham condition; p < 0.05 (N = 3–6 mice/group/time point). F) Representative images of Sirius Red histochemistry of interstitial collagen content. Scale bar=50 µm. G) Quantification of Sirius Red area as a percent of total muscle area. Data are presented as the fold-difference from the Vehicle Sham condition within each experimental paradigm. ‡ denotes significant difference for SC-Dep; † significant increase relative to Sham condition; p < 0.05 (N = 4–7 mice/group/time point). H) Plantaris muscle mass normalized to body weight. † denotes significant difference for surgery; * denotes significant difference between SC-WT and SC-Dep within the respective time point; p < 0.05 (N = 6–9 mice/group/time point). I) Quantification of the average cross-sectional area of the different fiber types. † denotes significant difference for surgery; p < 0.05 (N = 6–9 mice/group). Data presented as Mean ± SEM unless otherwise stated. Data for SC-WT and SC-Dep animals in Panels F and I has be recreated from previously published data (Fry et al., 2014) for comparison to the effects of SC-WTSA1 and –DepSA1. See also Figure S7.
MPC exosome-derived miR-206 regulates fibrogenic cell collagen expression through ribosomal binding protein 1
qRT-PCR miRNA microarray analysis was performed on RNA isolated from muscle Tcf4+ primary fibrogenic cells, MPCs and MPC exosomes to identify highly expressed miRNAs in MPC exosomes with potential to impact fibrogenic cell collagen expression. Criteria used for candidate miRNA identification were high expressed in both MPCs and MPC exosomes, and low expression in fibrogenic cells, so that if the putative miRNA were delivered from an MPC exosome to a muscle fibrogenic cell, it could have a physiological effect. Table 1 shows the list of miRNAs that meet the above criteria; the complete list of all profiled miRNAs from the 3 samples is presented in Supplemental Table 1. Not surprisingly, strong candidates included various muscle-specific miRNAs (Kim et al., 2006); in particular, miR-206 was highly abundant in both MPCs and MPC exosomes with a 10 Ct lower expression in muscle fibrogenic cells (Table 1). Further, miR-206 expression has been shown to increase 7 fold as satellite cells become activated (Cheung et al., 2012).
Table 1.
Highly expressed miRNA’s in MPC exosomes with MPC and fibroblast expression levels
| Ct Value | |||
|---|---|---|---|
| miR- | MPC Exosome | MPC | Fibroblast |
| 206 | 20.0 | 20.3 | 30.2 |
| 24 | 22.5 | 20.1 | 20.2 |
| 17 | 22.7 | 21.0 | 22.0 |
| 106a | 22.9 | 21.2 | 22.3 |
| 133a | 23.2 | 20.1 | 34.8 |
| 20a | 23.4 | 21.7 | 23.3 |
| 486 | 24.0 | 25.4 | 40.0 |
| 222 | 24.3 | 21.7 | 23.0 |
| 191 | 24.3 | 22.3 | 23.2 |
| 19b | 24.3 | 20.9 | 22.4 |
| 687 | 24.4 | 25.1 | 25.8 |
| 23 | 24.6 | 26.3 | 33.1 |
| 126 | 24.8 | 29.4 | 28.5 |
| 489 | 28.8 | 29.3 | 29.1 |
| 1 | 29.9 | 25.9 | 40.0 |
Muscle fibrogenic cells cultured with MPC exosomes had a 50% increase in the cellular content of miR-206 (Figure 3A), providing evidence for delivery of the muscle-specific miRNA to fibrogenic cells in vitro. TargetScan was used to generate a list of predicted and confirmed targets of miR-206 (Supplemental Table 2). Ribosomal binding protein 1 (Rrbp1) demonstrated a very strong context score, and is a master regulator of collagen biosynthesis (Ueno et al., 2010). Additionally, the expression of Rrbp1 was increased nearly 2 fold following 1 week of overload in satellite cell-depleted muscle (Supplemental Table 3), so we selected it for further study. Rrbp1 is highly expressed and necessary for secretory activity in cells such as fibroblasts (Benyamini et al., 2009; Ueno et al., 2010); knockdown of Rrbp1 levels leads to a dramatic decrease in procollagen biosynthesis in fibroblasts (Ueno et al., 2010). Following culture of fibrogenic cells with MPC exosomes, we found no change in Rrbp1 mRNA expression but did observe a decrease in Rrbp1 protein content (Figure 3B–C). However, direct transfection of a miR-206 mimic into muscle Tcf4+ fibrogenic cells resulted in a significant decrease in both Rrbp1 mRNA (Figure 3D) and protein content (Figure 3E). To demonstrate direct miR-206 regulation of Rrbp1, we cloned the Rrbp1 3’ untranslated region (UTR), containing the predicted miR-206 binding site, downstream of the luciferase gene. Luciferase activity was effectively down-regulated following transfection of miR-206 mimic into 3T3 fibroblasts (Figure S3A). Mutation of the miR-206 seed sequence within the Rrbp1 3’-UTR (Rrbp1m, Figure S3A) resulted in no decrement in luciferase activity following transfection with miR-206 mimic, demonstrating the specificity of the predicted miR-206 binding site. A schematic of miR-206 regulation of Rrbp1 expression is shown in Figure S3B with mutated nucleotides bolded.
Figure 3. Myogenic progenitor cell (MPC) exosome-derived miR-206 regulates fibrogenic cell collagen expression through ribosomal binding protein 1 (Rrbp1).
Primary fibrogenic cell A) miR-206 content, B) Rrpb1 mRNA abundance and C) Rrbp1 protein content. * significant effect of MPC exosomes; p < 0.05. Primary fibrogenic cell D) Rrbp1 mRNA content and E) protein content. * denotes significant effect of miR-206 transfection; p < 0.05. F) MPC and MPC exosome miRNA content following transfection with antagomiR-206. Primary fibrogenic cell G) Rrbp1 mRNA abundance, H) Rrbp1 protein content and I) collagen and fibronectin mRNA abundance. See also Figure S3 and S6 and Table S1–S3. * denotes significant effect of miR-206 depletion by antagomiR (anti-206) on MPC exosome effects; p < 0.05 (For all experiments, N = 3 independent primary cell isolates studied in duplicate). All data presented as Mean ± SEM.
The transfection of miR-206 mimic into fibrogenic cells negatively regulated the expression of Col1a2, Col3a1, Col6a2 and fibronectin (Figure S3C). We then sought to determine if decreased Rrbp1 content would negatively regulate fibrogenic cell collagen mRNA levels. Following transfection of Rrbp1 siRNA, we achieved significant knockdown of both Rrbp1 transcript (Figure S3D) and protein (Figure S3E) in fibrogenic cells. The knockdown of Rrbp1 resulted in significantly decreased Col1a2 and Col6a2 expression (Figure S3F), with the decrease in Col3a1 expression nearly reaching significance (P = 0.06; Figure S3F).
Knockdown of miR-206 abolishes the effect of MPC exosomes on fibrogenic cell Rrbp1 and ECM gene expression
To demonstrate the specificity of MPC exosome-derived miR-206 in the regulation of fibrogenic cell ECM biosynthesis, we transfected a miR-206 antago-miR into MPCs, achieving efficient knockdown of endogenous MPC miR-206 levels, while not affecting the expression of other miRNAs (Figure 3F). Exosomes isolated from miR-206-depleted MPCs also demonstrated strong knockdown of miR-206 (Figure 3F). Co-culture of fibrogenic cells with miR-206-depleted MPC exosomes did not affect Rrbp1 mRNA expression (Figure 3G), but eliminated the down regulation of Rrbp1 protein expression, providing evidence for the specificity of miR-206 in mediating the effects of MPC exosomes (Figure 3H). Co-culture of miR-206-depleted MPC exosomes with fibrogenic cells resulted in higher expression of Col1a2, 3a1 and 6a2, as well as fibronectin compared to MPC exosomes (Figure 3I), providing further evidence for the specific role of MPC exosome-derived miR-206 in the regulation of fibrogenic cell ECM expression.
Satellite cell-depletion is associated with reciprocal expression changes in miR-206 and Rrbp1 levels, coupled with increased collagen expression
We next sought to determine whether the miR-206/Rrbp1 regulatory mechanism identified in vitro was negatively impacted in our in vivo mouse model of satellite cell depletion. Using the Pax7-DTA mouse strain, we hypothesized that miR-206 and Rrbp1 would be differentially expressed during acute mechanical overload with satellite cell-depletion. First, to determine the temporal expression changes of Rrbp1 and a panel of collagen transcripts, we analyzed a microarray database consisting of a high-density of sampling time points following mechanical overload in C57Bl6 mice (GSE47098) (Chaillou et al., 2013). As shown in Figure S4, the peak expression change for all abundant collagens occurred 7 to 10 days following mechanical overload. Similarly, Rrbp1 expression also peaked 7 days following mechanical overload (Figure S4 inset). We previously reported microarray data from Pax7-DTA mice demonstrating the expression of various collagen isoforms is significantly higher 7 days following mechanical overload in satellite cell-depleted animals (Fry et al., 2014). Thus, we focused on acute time points following mechanical overload to determine whether the pattern of miR-206 and Rrbp1 expression was consistent with the interaction validated in fibrogenic cells.
The satellite cell pool was either depleted (SC-Dep) or maintained (SC-WT) prior to mechanical overload and plantaris was collected after one week (SA1) or two weeks (SA2) of synergist ablation (Pax7+ satellite cell counts for the muscles are presented in Figure S1 and Tcf4+ fibrogenic cell counts in Figure 1). In response to mechanical overload, miR-206 increased by 3-fold at SA1 and 2.7-fold at SA2 in SC-WT animals; however this increase was completely abolished in the SC-Dep animals (Figure 4A). These findings suggest that not only is miR-206 skeletal muscle-specific, but in addition, is highly enriched in satellite cells. Next, we sought to determine whether the loss of miR-206 expression in SC-Dep animals resulted in a concomitant increase in Rrbp1 levels. Rrbp1 mRNA levels increased in both SC-WT and SC-Dep animals in response to mechanical overload; however, SC-Dep animals demonstrated significantly greater Rrbp1 mRNA expression levels (~48% at SA1 and ~29% at SA2) relative to SC-WT (Figure 4B). At the protein level, Rrbp1 was ~247% higher in SC-Dep animals (Figure 4C), consistent with our transfection experiments indicating that miR-206 primarily regulates Rrbp1 at through translational inhibition (Figure 3B–E).
Figure 4. Depletion of satellite cells results in lower miR-206 expression and higher Rrbp1 and collagen expression in vivo during the early phases of mechanical overload.
A) miR-206 expression levels. * denotes significant difference between SC-WT and SC-Dep within the respective time point; † significant increase relative to Sham condition within that respective group; p < 0.05 (N = 6–11 animals/group/time point). B), Rrbp1 mRNA levels. ‡ denotes significant difference for SC-Dep group; † denotes significant increase relative to sham; p < 0.05 (N = 6–11 animals/group/time point). C) Quantification of Rrbp1 protein levels at SA1 using western blot. * denotes significant difference between SC-WT and SC-Dep; † denotes significant difference for surgery; p <0.05 (N = 7–8 animals/group). D), Col1a2, E), Col3a1 and F), Col12a1 mRNA levels. ‡ denotes significant difference for SC-Dep group; p < 0.05; Col3a1 trend for significant main effect; P = 0.06 (N = 7–11 animals/group/time point). Data presented as Mean ± SEM. See also Figure S4 and S5.
Our findings suggest that the loss of miR-206 upon depletion of satellite cells relieves the repression of miR-206 on Rrbp1 during mechanical overload. In vitro, we demonstrated that the specific knockdown of Rrbp1 in fibrogenic cells decreases collagen mRNA levels (Figure S3F). Therefore, we hypothesized that this increase in Rrbp1 levels at SA1 and SA2 in SC-Dep animals would also be associated with overexpression of collagen mRNA levels, and further, providing a potential mechanism underlying the aberrant ECM accumulation in mechanically-overloaded, satellite cell-depleted muscle (Fry et al., 2014). We reasoned that this substantial accumulation of collagen would likely be a result of mis-regulated expression of the most abundant collagen isoforms. Thus, we chose to focus on Col1a2, Col3a1 and Col12a1 which are highly abundant in skeletal muscle and demonstrated a significant upregulation in response to mechanical overload (Figure S2). Consistent with our in vitro (Figure S3F) and microarray findings (Fry et al., 2014), increased Rrbp1 expression in SC-Dep animals is associated with higher expression of Col1a2, Col3a1 and Col12a1 following mechanical overload compared to SC-WT animals (Figure 4D–F). No effect of satellite cell depletion was observed on collagen expression levels in sham-treated animals (Figure S5), which is in line with the dramatic remodeling of the ECM that occurs during the first week of overload (Figure S4). These findings are consistent with the model that an activated satellite cell/MPC-mediated miR-206/Rrbp1 interaction in muscle fibrogenic cells governs the global expression of collagens to facilitate appropriate ECM remodeling. This interaction between MPCs and muscle fibrogenic cells has been suggested in the remodeling process that follows muscle injury (Murphy et al., 2011). Loss of satellite cells during muscle regeneration results in mis-regulation of muscle fibroblasts, resulting in a pronounced increase of connective tissue within the muscle (Murphy et al., 2011). We propose that this is a result of a miR-206/Rrbp1-dependent mechanism, as miR-206 is also dramatically upregulated in models of muscle regeneration (Liu et al., 2012).
Tamoxifen treatment of Pax7-DTA mice at SA1 effectively depletes MPCs and attenuates nuclear accretion
It is clear this model of mechanical overload results in significant ECM remodeling, as even SC-WT animals experience increased collagen expression and ECM accumulation (Fry et al., 2014). Peak expression changes for miR-206, Rrbp1, Col1a2, Col3a1 and Col12a1 all occurred at Day 7 (SA1) following mechanical overload (Figure 4A–B, D–F; Figure S4), therefore, we reasoned that perhaps the regulation of Rrbp1 by miR-206 is most critical during this acute period of ECM remodeling. Results from the Pax7-cKO Dicer mouse also suggest that the presence of activated satellite cells early during growth can have a significant impact on long term growth and ECM accumulation (see Figures 2G and H). We hypothesized that the activation of satellite cells to generate MPCs and their secretion of exosomes containing miR-206 is critical during the first week of mechanical overload, and their presence only during this time period would be sufficient to facilitate appropriate ECM remodeling and prevent excessive accumulation of ECM.
To test the hypothesis that activated satellite cells/MPCs are only necessary during the first week of mechanical overload in order to properly regulate collagen expression, Pax7-DTA animals were mechanically overloaded, followed by depletion of the satellite cell pool at SA1 (SC-DepSA1) (Figure 5A). Treatment with tamoxifen during a period when satellite cells are activated and proliferating (Figure S1) still allowed for the significant depletion of the satellite cell pool at SA8 (Figure 5B and C). Importantly, despite the increase in Pax7+ cell number at one week, minimal fusion had occurred, resulting in significantly less nuclear accretion at SA8 in SC-DepSA1 animals relative to SC-WTSA1 (Figure 5D). These results are consistent with previous work demonstrating that minimal nuclear accretion occurs within the first 7 days of mechanical overload (Bruusgaard et al., 2010). Using this paradigm, we tested whether normal satellite cell activation and proliferation during onset of ECM remodeling was sufficient to rescue the negative hypertrophic phenotype observed when satellite cells are absent (Fry et al., 2014).
Activated satellite cells/MPCs are required for appropriate ECM remodeling during the acute phase of mechanical overload to facilitate myofiber growth
Our data indicate that depletion of activated satellite cells during the early onset of mechanical overload (i.e. SA1) results in mis-regulated expression of various collagen isoforms (Figure 4D–F). We hypothesized that the presence of activated satellite cells/MPCs specifically during this period would permit their regulation of ECM remodeling, resulting in robust hypertrophy of the fibers without increased fibrosis with prolonged overload.
In Figure 5E, when satellite cells are depleted prior to the onset of hypertrophy there is a significant increase in ECM content at SA8 (SC-WT vs SC-Dep). However, when activated satellite cells remain during the first week of overload (SA1), and then are depleted, the excessive ECM accumulation is prevented (SC-WTSA1 vs SC-DepSA1). Using Sirius Red staining to quantify collagen-specific content (Figure 5F), we found that depletion of satellite cells prior to mechanical overload surgery resulted in increased collagen content, relative to the normal increase that occurs in response to mechanical overload (Figure 5G). Conversely, allowing activation and expansion of the satellite cell pool during the first week, followed by depletion, was effective at preventing excessive collagen accumulation observed when satellite cells are depleted prior to overload surgery (Figure 5G). Intriguingly, the presence of activated satellite cells during the first week of overload did not prevent the increase in Tcf4+ cell content following 8 weeks of overload (Figure S7). These data are similar to those presented in Figure 1H, and provide evidence that activated satellite cells regulate Tcf4+ fibrogenic cell expansion throughout the overload period. Taken together, these findings provide strong evidence that activated satellite cells/MPCs are actively involved in regulating their extracellular niche during the early phases of mechanical overload.
Next, we wanted to determine whether rescuing the fibrotic phenotype facilitated robust myofiber hypertrophy, which we demonstrated is attenuated with elevated ECM content (Fry et al., 2014). By rescuing the fibrotic phenotype, hypertrophic growth at the whole muscle level was restored (Figure 5H). At the level of the myofiber, when activated satellite cells/MPCs were depleted after one week of overload (SA1), hypertrophy occurred to the same degree as those animals with their full complement of satellite cells (SC-WTSA1 SA8 vs SC-DepSA1 SA8) (Figure 5I). These finding suggest that the over-accumulation of ECM, as opposed to the lack of myonuclear accretion, may ultimately limit myofiber hypertrophy in satellite cell-depleted muscle.
Discussion
During muscle adaptation, the ECM undergoes substantial remodeling which influences satellite cell activity (Urciuolo et al., 2013). The biochemical composition and stiffness of the extracellular niche can directly impact satellite cell self-renewal and expansion (Gilbert et al., 2010a; Montarras et al., 2005; Urciuolo et al., 2013). Satellite cells contribute to the composition of the local microenvironment (El Fahime et al., 2000; Guerin and Holland, 1995), and our data show that MPC-mediated regulation of the muscle ECM is essential during muscle hypertrophy. Direct modulation of fibrogenic cell ECM production allows activated satellite cells/MPCs to exert an additional measure of “self-regulation” of their own fate (Kuang et al., 2008).
Functional overload of the plantaris induces robust remodeling of the ECM (Chaillou et al., 2013), and during the first week of overload, ECM remodeling displays a similar expression profile as that of freeze injury to muscle (Warren et al., 2007). Although early ECM remodeling during regeneration and hypertrophy are similar, hypertrophy and regeneration are distinct processes, with different satellite cell requirements (McCarthy et al., 2011). It is interesting to speculate a role for MPC exosomes and miR-206 specifically in the remodeling of the ECM during regeneration; expansion of the ECM occurs following BaCl2 injection in satellite cell-depleted muscle that is associated with expansion of Tcf4+ fibrogenic cells (Murphy et al., 2011). miR-206 is known to be involved in the regulation of satellite cell behavior and muscle regeneration (Chen et al., 2010; Liu et al., 2012), with the loss of miR-206 negatively affecting regeneration (Liu et al., 2012). However, evidence also exists that miR-206 is dispensable for muscle regeneration (Boettger et al., 2014). In the current study, we observed an expansion of Tcf4+ cells with overload that was unaffected by satellite cell depletion, suggesting that ECM synthesis increased on a per-cell basis in the absence of MPC-derived miR-206.
Regulation of fibrogenic cell collagen and fibronectin expression was accomplished through modulation of Rrbp1 by MPC exosome-derived miR-206 in vitro. Although over-expression of miR-206 in fibrogenic cells resulted in decreased Rrbp1 mRNA and protein levels, co-culture and exosome delivery of miR-206 appeared to regulate Rrbp1 primarily at the level of translation. Rrbp1 is involved with the localization of a subset of mRNAs to the endoplasmic reticulum in a ribosome-independent manner to assist in the translation of mRNAs and secretion of various protein products (Cui et al., 2012). Our findings show that Rrbp1 regulates collagen expression at both the mRNA and protein levels. Previous work from Ueno and colleagues showed Rrbp1-mediated regulation of collagen protein expression (Ueno et al., 2010). While that report showed only a trend for decreased collagen and fibronectin mRNA abundance following Rrbp1 knockdown, results of their knockdown experiments were highly variable, which may have masked their ability to detect a statistically significant effect at the mRNA level (Ueno et al., 2010). An additional difference between our studies is that our results explore regulation of physiological versus chemically-induced collagen expression (Ueno et al., 2010). Differences also exist in the time fibroblasts were harvested following transfection for RNA analyses. While the precise mechanism through which Rrbp1 regulates collagen mRNA expression remains to be determined, it is clear that Rrbp1 is capable of affecting collagen mRNA stability and translation following overload in muscle.
In the current study, we utilized mechanical overload as a model to delineate the role of MPC-mediated ECM remodeling during adaptation. Significant remodeling of the ECM occurs during the first week of overload, and both the Pax7-DTA and Pax7-Dicer cKO mice show that MPCs are crucial during this first week, when collagen mRNA accumulation begins to rise, in order to limit ECM deposition long term. During later stages of hypertrophy, MPC activity appears dispensable, suggesting alternative regulatory mechanisms contribute to ECM homeostasis during later stages of growth. Currently, it is unclear whether MPC-regulation of the extracellular environment is directed at permitting myofiber hypertrophy, or rather, that effective hypertrophy is secondary to maintaining a symbiotic relationship between the extracellular niche and MPCs themselves. Satellite cells are known to regulate their own microenvironment via intrinsic production of ECM components (Bentzinger et al., 2013; Brohl et al., 2012), with our results indicating a mechanism through which activated satellite cells/MPCs possess the capability to modulate collagen expression in neighboring fibrogenic cells. A model of this is presented in Figure 6.
Figure 6. Model of the interaction between satellite cells and muscle fibrogenic cells during the acute phase of ECM remodeling and myofiber hypertrophy.
Satellite cells become activated by mechanical overload, proliferate and secrete exosomes into the extracellular niche. The exosomes containing miRNA, including miR-206, are released from the activated satellite cells/MPCs, which then dock and release their contents into nearby muscle fibrogenic cells. MPC-enriched miR-206 binds the Rrbp1 mRNA 3’-UTR, leading to a reduction in both mRNA and protein content, which is associated with reduced collagen mRNA expression in muscle fibrogenic cells. This regulatory mechanism is imperative during the first week of mechanical overload, the time when ECM remolding and collagen expression is the highest, in order to facilitate appropriate remodeling and prevent fibrosis. The absence of activated satellite cells during this critical remodeling period results in unregulated expression of Rrbp1 and collagen, resulting in increased ECM deposition and attenuated myofiber growth. Dashed inhibitory line represents unspecified Rrbp1 negative regulation of collagen mRNA expression.
The current study shows MPC-mediated regulation of the muscle extracellular matrix during remodeling, and the mechanism of this interaction with fibrogenic cells is further explored using various in vitro systems. We are limited in our interpretation of these findings without clear demonstration of MPC exosome-fibrogenic cell interaction in vivo. Difficulty tracking exosome delivery over the eight week overload period represents one of the challenges of in vivo modeling. We are confident however that both our in vivo and in vitro data support our proposed mechanism, and offer insight into the paracrine regulation of the extracellular environment by MPCs.
In clinical conditions characterized by reduced satellite cell activity, such as muscular dystrophy and aging, excessive fibrosis occurs, which in turn impairs muscle adaptation (Alnaqeeb et al., 1984; Brack et al., 2007; Mann et al., 2011; Stedman et al., 1991). Furthermore, these conditions are associated with an increased stiffness of the muscle (Rosant et al., 2007; Stedman et al., 1991), which negatively affects satellite cell behavior (Engler et al., 2004). Satellite cells are direct participants in the development of a fibrotic muscle environment (Alexakis et al., 2007). Intriguingly, the depletion of miR-206 exacerbates the dystrophic phenotype in mdx mice (Liu et al., 2012), offering further support for our MPC-miR-206 model of muscle ECM remodeling. Finally, the genetic depletion of satellite cells exacerbates the age-related increase in muscle ECM (Fry et al., 2015; Lee et al., 2015), providing further support that activated satellite cells/MPCs are key participants in the remodeling of their extracellular environment. We propose that MPCs directly regulate their surrounding ECM composition during remodeling via exosome-mediated regulation of fibrogenic cell ECM production, providing a mechanism to explain the fibrotic pathogenesis associated with other conditions (i.e. muscular dystrophy, aging) in which satellite cell activity is compromised.
Experimental Procedures
Animal Studies
All animal procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals, as approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Mice were housed in a temperature- and humidity-controlled room and maintained on a 14:10 hour light: dark cycle with food and water ad libitum. All experiments were performed on ~4-month-old mice. To induce Cre recombination, mice were administered an intraperitoneal injection of tamoxifen (2 mg/d), or vehicle control (15% ethanol in sunflower seed oil), for 5 consecutive days. The Pax7-Dicer strain was used to deplete satellite cells of mature microRNAs, and the Pax7-DTA strain was used to specifically deplete the satellite cell pool.
Cell Culture Studies
Detailed procedures have been published previously (Fry et al., 2014; Hidestrand et al., 2008). MPCs were cultured in growth media + (GM+; DMEM [Mediatech/Corning, Manassas, VA] + 20% fetal bovine serum [Atlanta Biologicals, Flowery Branch, GA] + basic Fibroblast Growth Factor [5ng/ml] [Millipore, Temecula, CA]) and fibrogenic cells in GM- (DMEM + 20% fetal bovine serum). Fibrogenic cells were characterized by their near-uniform expression of Tcf4 (Figure S6A–D). MPCs were characterized by their expression of MyoD, with greater than 97% of cells expressing MyoD (Figure S6E–H). Conditioned media was collected following a 24 hr conditioning period. Exosomes were isolated from MPC CM with the ExoQuick-TC kit (Systems Biosciences, San Francisco, CA) according to manufacturer instructions. Transfection of primary fibrogenic cells and NIH 3T3 fibroblasts was accomplished using Lipofectamine 2000 (Life Technology) according to manufacturer instructions. Primary fibrogenic cells were harvested for RNA and protein 24 hr following transfection, and luciferase activity of 3T3 cells was measured 24 hr following transfection.
Statistical analysis
A two-way ANOVA, one-way ANOVA or student’s paired t-test was performed to determine whether a significant interaction existed between factors for each dependent variable under consideration. Data that demonstrated a non-normal distribution was log-transformed. Significance was set at P ≤ 0.05.
Supplementary Material
Highlights.
Myogenic progenitor cells (MPCs) regulate the muscle extracellular matrix
MPCs regulate skeletal muscle fiber hypertrophy independent of fusion
miR-206 in MPC exosomes regulates fibrogenic cell Rrbp1 and collagen expression
MPCs regulate ECM remodeling within 1 week to facilitate fiber growth
Acknowledgments
The authors would like to thank Janna Jackson, Grace Walton, Alexander Alimov, Samantha Michaelis, Angel Ho and Sarah White for their technical assistance. Research was supported by NIH grant AR065337 and the Jeane B. Kempner Postdoctoral Scholar Award to C.S.F.; NIH grants AG034453 to C.A.P. and AR060701 and AG049806 to C.A.P. and J.J.M.; and the NIH National Center for Advancing Translational Sciences, through Grant UL1TR000117. C.S.F. is a KL2 scholar supported by the UTMB Claude D. Pepper Older Americans Independence Center NIH/NIA grant P30 AG024832. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, C.S.F., T.J.K., J.J.M. and C.A.P.; Methodology, C.S.F., T.J.K., J.J.M. and C.A.P.; Investigation, C.S.F., T.J.K., K.K.; Writing – Original Draft, C.S.F., T.J.K.; Writing – Review & Editing, C.S.F., T.J.K., K.K., J.J.M. and C.A.P.; Funding Acquisition, C.S.F., J.J.M. and C.A.P.; Resources, J.J.M. and C.A.P.; Supervision, J.J.M. and C.A.P.
The authors declare no conflicts of interest.
References
- Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat. 1984;139(Pt 4):677–689. [PMC free article] [PubMed] [Google Scholar]
- Barberi L, Scicchitano BM, De Rossi M, Bigot A, Duguez S, Wielgosik A, Stewart C, McPhee J, Conte M, Narici M, et al. Age-dependent alteration in muscle regeneration: the critical role of tissue niche. Biogerontology. 2013;14:273–292. doi: 10.1007/s10522-013-9429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamini P, Webster P, Meyer DI. Knockdown of p180 Eliminates the Terminal Differentiation of a Secretory Cell Line. Mol Biol Cell. 2009;20:732–744. doi: 10.1091/mbc.E08-07-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T, Wust S, Nolte H, Braun T. The miR-206/133b cluster is dispensable for development, survival and regeneration of skeletal muscle. Skelet Muscle. 2014;4:23. doi: 10.1186/s13395-014-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Braghetta P, Ferrari A, Fabbro C, Bizzotto D, Volpin D, Bonaldo P, Bressan GM. An enhancer required for transcription of the Col6a1 gene in muscle connective tissue is induced by signals released from muscle cells. Exp Cell Res. 2008;314:3508–3518. doi: 10.1016/j.yexcr.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Brohl D, Vasyutina E, Czajkowski MT, Griger J, Rassek C, Rahn HP, Purfurst B, Wende H, Birchmeier C. Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev Cell. 2012;23:469–481. doi: 10.1016/j.devcel.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A. 2010;107:15111–15116. doi: 10.1073/pnas.0913935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344:259–271. doi: 10.1016/j.ydbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol. 2013;115:1065–1074. doi: 10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Tao YZ, Li JA, Deng ZL, Yan Z, Xiao XA, Wang DZ. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190:867–879. doi: 10.1083/jcb.200911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524-U247. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Cui XYA, Zhang H, Palazzo AF. p180 Promotes the Ribosome-Independent Localization of a Subset of mRNA to the Endoplasmic Reticulum. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258:279–287. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu HL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28:1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010a;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science. 2010b;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev Dyn. 1995;202:91–99. doi: 10.1002/aja.1002020109. [DOI] [PubMed] [Google Scholar]
- Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- Hidestrand M, Richards-Malcolm S, Gurley CM, Nolen G, Grimes B, Waterstrat A, Van Zant G, Peterson CA. Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J Gerontol A Biol Sci Med Sci. 2008;63:566–579. doi: 10.1093/gerona/63.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaariainen M, Jarvinen T, Jarvinen M, Rantanen J, Kalimo H. Relation between myofibers and connective tissue during muscle injury repair. Scand J Med Sci Sports. 2000;10:332–337. doi: 10.1034/j.1600-0838.2000.010006332.x. [DOI] [PubMed] [Google Scholar]
- Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol. 2006;174:677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M, Magnusson P, Krogsgaard M, Moller JB, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006;208:445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a Activates the Planar Cell Polarity Pathway to Drive the Symmetric Expansion of Satellite Stem Cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Fry CS, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Aged Muscle Demonstrates Fiber-Type Adaptations in Response to Mechanical Overload, in the Absence of Myofiber Hypertrophy, Independent of Satellite Cell Abundance. J Gerontol A Biol Sci Med Sci. 2016;71:461–467. doi: 10.1093/gerona/glv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Williams AH, Maxeiner JM, Bezprozvannaya S, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J Clin Investig. 2012;122:2054–2065. doi: 10.1172/JCI62656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosant C, Nagel MD, Perot C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp Gerontol. 2007;42:301–308. doi: 10.1016/j.exger.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Fitch JM, Linsenmayer TR, Mayne R. Fibroblasts promote the formation of a continuous basal lamina during myogenesis in vitro. J Cell Biol. 1986;102:740–747. doi: 10.1083/jcb.102.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Li Y, Foster W, Fukushima K, Badlani N, Adachi N, Usas A, Fu FH, Huard J. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- Shea KL, Xiang WY, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 Regulates Reversible Quiescence of a Self-Renewing Adult Muscle Stem Cell Pool during Regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Ueno T, Tanaka K, Kaneko K, Taga Y, Sata T, Irie S, Hattori S, Ogawa-Goto K. Enhancement of Procollagen Biosynthesis by p180 through Augmented Ribosome Association on the Endoplasmic Reticulum in Response to Stimulated Secretion. J Biol Chem. 2010;285:29941–29950. doi: 10.1074/jbc.M109.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:U654–U672. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Warren GL, Summan M, Gao X, Chapman R, Hulderman T, Simeonova PP. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol. 2007;582:825–841. doi: 10.1113/jphysiol.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zhang RZ, Sabatelli P, Chu ML, Bonnemann CG. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: Implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol. 2008;67:144–154. doi: 10.1097/nen.0b013e3181634ef7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.