Figure 1.
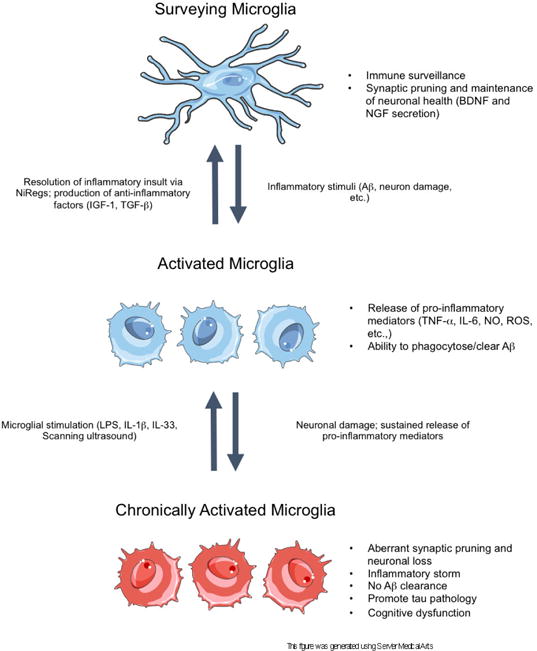
A schematic of acute and chronic microglial activation in the context of Alzheimer’s disease. In the surveillance state, microglia sample the brain parenchyma to detect anomalous materials, while concomitantly secreting anti-inflammatory factors and pruning unnecessary or weak synapses to support neuronal health. Disturbances in brain homeostasis, such as detection of Aβ, result in the activation of microglia. In the activated state, microglia release pro-inflammatory mediators to recruit other cells to the insult and to phagocytose the detected insult. When the insult is cleared, the inflammatory response resolves via NiReg (Neuroimmune regulatory proteins) activity, and microglia secrete anti-inflammatory factors to promote tissue repair and growth. However, in neurodegenerative diseases, the sustained release of pro-inflammatory mediators, as well as increasing neuronal death, drives the cells into a state of chronic activation. In this state, the homoeostatic functioning of microglia is lost, resulting in an accumulation of aggregated proteins, the stripping of synapses from neurons, as well as further neuronal loss. Importantly, in this reactive state, microglia can be stimulated with certain proteins or methods to induce microglial phagocytosis of Aβ, thereby restoring the phagocytic ability of microglia.
