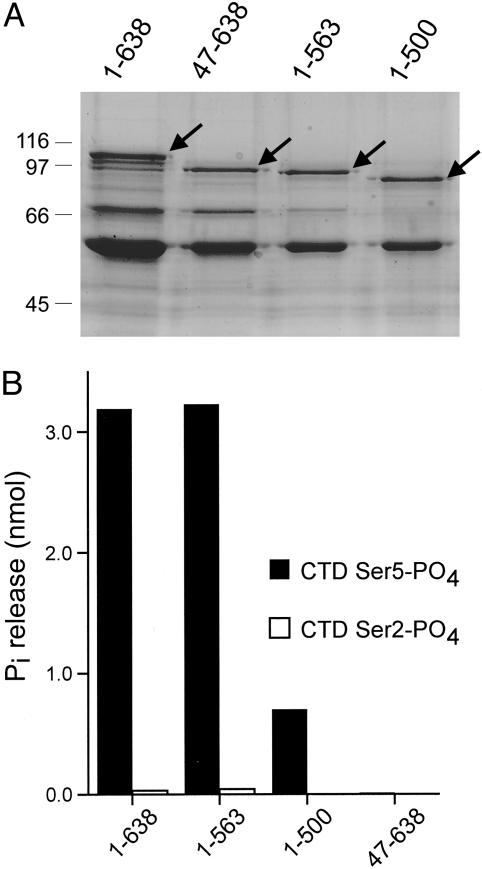
Fig. 2.
Effects of N-terminal and C-terminal deletions on AtCPL1 CTD phosphatase activity. (A) GST-CPL1 fusions. Aliquots of the affinity-purified GST-AtCPL1 fusion proteins CPL11-638, CPL147-638, CPL11-563, and CPL11-500 containing ≈0.1 μg of the respective GST-CPL1 fusion polypeptides were analyzed by SDS/PAGE. The Coomassie blue-stained gel is shown. The positions and sizes (kDa) of marker proteins are indicated on the left. The polypeptides corresponding to the GST-CPL1 fusions are indicated by arrows. (B) CTD phosphatase activity. Reaction mixtures (25 μl) containing 50 mM Tris-acetate (pH 5.5), 10 mM MgCl2, either 32 μM CTD Ser-5-PO4 phosphopeptide (YSPTSPS)4 (containing 3.2 nmol of input Ser-PO4) or 36 μM CTD Ser-2-PO4 phosphopeptide (YSPTSPS)4 (containing 3.6 nmol of input Ser-PO4), and ≈0.1 μg of the GST-CPL1 fusion polypeptides as specified were incubated for 60 min at 37°C. Representative results from three independent experiments are shown.