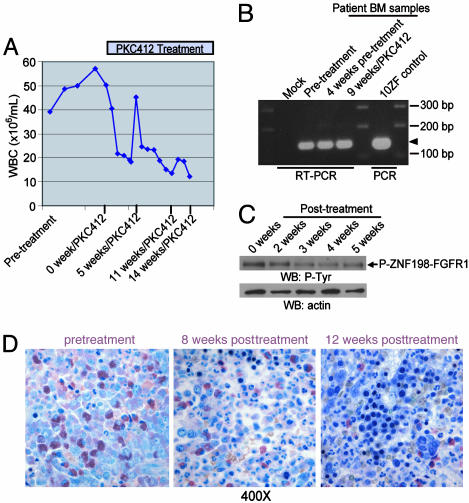
Fig. 5.
Efficacy of PKC412 in treating t(8:13)p11;q12)-associated myeloproliferative disorder in humans. (A) Progressive reduction in peripheral blood leukocyte count before and after initiation of PKC412 therapy. The transient increase 5 weeks posttreatment was associated with a bolus of steroids given to treat an allergic reaction to i.v. contrast. (B) RT-PCR analysis of RNA derived from patient bone marrow samples before and during therapy with PKC412. A fragment of a 154-bp unique ZNF198-FGFR1 fusion sequence was amplified and is indicated by an arrowhead. A mock RT-PCR reaction with water instead of RNA template and a PCR reaction with plasmid-encoding 10ZF as a template were included as controls. (C) Western blot analysis of patient samples before and during therapy with PKC412 demonstrated progressive reduction in phosphotyrosine content of ZNF198-FGFR1. (D) Histopathologic examination by a standard Giemsa stain on the patient samples, which are from Zenker's fixed bone marrow core biopsy specimens before therapy with PKC412, shows a hypercellularity of immature myeloid lineage cells, increased immature granulocytic forms including eosinophils, and <5% blast forms (Left). Subsequent examination after initiation of therapy showed progressive increase in maturity in the myeloid lineage and normalization of the myeloid-toerythroid ratio with corresponding increases in erythropoietic and megakaryocyte lineage cells.