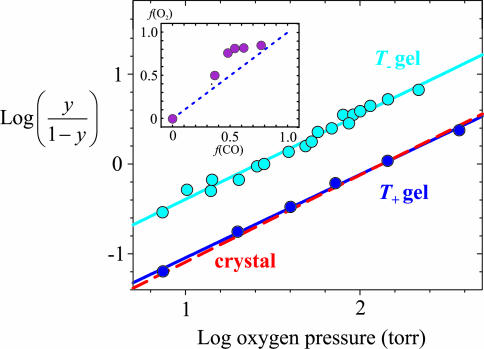
Fig. 2.
O2-binding curves. Shown is a Hill plot of O2-binding curves for T- gel (100 mM Hepes buffer/1 mM EDTA, pH 7.0; cyan circles); T+ gel (100 mM Hepes buffer/10 mM inositol hexaphosphate/2 mM bezafibrate/200 mM chloride ions/1 mM EDTA, pH 7.0; blue circles); and single crystal in T quaternary structure, red dashed line. Data is from Mozzarelli et al. (19). y, fractional saturation of the hemes. The continuous lines through the data yield p50 = 26 ± 1 torr and n = 0.95 ± 0.03 for T-, and p50 = 134 ± 5 torr and n = 0.93 ± 0.03 for T+. The corresponding values for the crystal are p50 = 135 ± 1 torr and n = 0.97 ± 0.03. (Inset) Fraction of liganded r subunits in T quaternary structure (T- gels) from oxygen-binding curves [f(O2)] versus fraction of liganded r subunits in T quaternary structure from CO photolysis experiments [f(CO)], determined at different pH values and in the presence or absence of chloride ions. The former is calculated from f(O2) = {1 - [p50(T-)/p50(T+)]}. The p50 of the T+ gels is independent of pH between 6.6 and 7.6.