Abstract
Oxidative stress plays a role in UV-induced melanoma, which may arise from melanocytic nevi. We investigated whether oral administration of the antioxidant N-acetylcysteine (NAC) could protect nevi from oxidative stress in vivo in the setting of acute UV exposure. The minimal erythemal dose (MED) was determined for 100 patients at increased risk for melanoma. Patients were randomized to receive a single dose (1200 mg) of NAC or placebo, in double-blind fashion, and then one nevus was irradiated (1–2 MED) using a solar simulator. One day later, the MED was re-determined and the irradiated nevus and a control un-irradiated nevus were removed for histologic analysis and examination of biomarkers of NAC metabolism and UV-induced oxidative stress. Increased expression of 8-oxoguanine, thioredoxin reductase-1, and γ-glutamylcysteine synthase modifier subunit were consistently seen in UV-treated compared to unirradiated nevi. However, no significant differences were observed in these UV-induced changes or in the pre- and post-intervention MED between those patients receiving NAC vs. placebo. Similarly, no significant differences were observed in UV-induced changes between subjects with germline wild-type vs. loss of function mutations in the melanocortin-1 receptor. Nevi showed similar changes of UV-induced oxidative stress in an open-label post-trial study in 10 patients who received NAC 3 h before nevus irradiation. Thus a single oral dose of NAC did not effectively protect nevi from UV-induced oxidative stress under the conditions examined.
Keywords: N-acetylcysteine, chemoprevention, melanoma, antioxidant, nevi, UV
Introduction
Melanoma incidence continues to increase rapidly in the U.S. (1), and despite recent therapeutic advances (2) most patients with metastatic disease will ultimately succumb to it (3). Melanoma often arises from melanocytic nevi (i.e. moles) (4), which frequently are found on sun-exposed areas (5). Ultraviolet (UV) radiation in sunlight (6) or from tanning beds (7) is the major environmental risk factor for melanoma. There is currently no proven effective primary prevention strategy, although education and regular skin screening may facilitate early detection (8) and use of sunscreen may reduce risk (9).
Reactive oxygen species (ROS) are generated in the skin upon UV exposure (10). Oxidative stress caused by elevated ROS can damage intracellular proteins and lipids (11) and lead to formation of oxidative DNA lesions such as 8-oxoguanine (8-OG) that can result in oncogenic mutations if not repaired prior to DNA replication (12). Glutathione (GSH) is the major thiol reductant in cells that, along with thioredoxin reductases (TR) and other endogenous antioxidants, can quench ROS and mitigate cellular damage (13). There is a good deal of correlative evidence suggesting that one link between UV radiation and melanoma may lie in the generation of oxidative damage (14), and melanoma has been referred to as a “reactive oxygen-driven tumor” (15). Germline variants in the melanocortin-1 receptor (MC1R), which regulates UV-induced ROS production and metabolism in melanocytes (16), are relatively common, and several are associated with melanoma predisposition independent of UV exposure (17). Finally, we reported that the antioxidant N-acetylcysteine (NAC) can delay tumor development in an animal model of UV-induced melanoma (18).
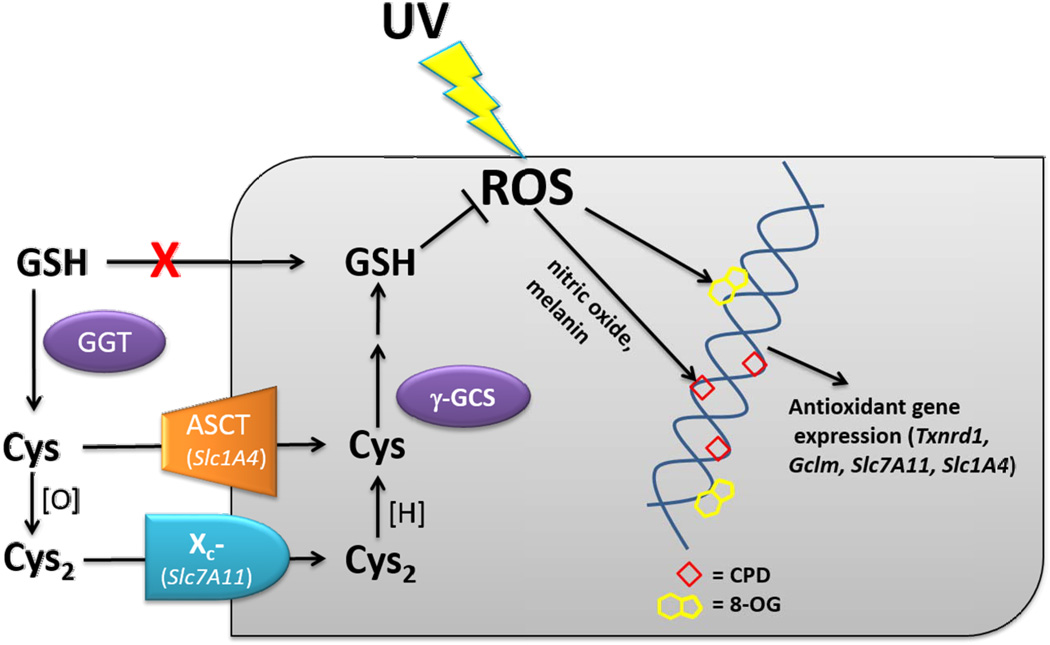
NAC is Food and Drug Administration-approved for acetaminophen toxicity (19), and has subsequently been used to preserve lung function in patients with idiopathic pulmonary fibrosis (20) and to prevent contrast-medium-induced nephropathy in patients undergoing angioplasty (21). There are over 100 studies in progress investigating NAC for other applications, including schizophrenia, heart failure, Parkinson’s disease, and bipolar disorder (22). NAC is an orally bioavailable antioxidant that is pro-drug form of L-cysteine (Cys). Following metabolism of NAC, the enzyme γ-glutamylcysteine synthase (γ-GCS) catalyzes the coupling of Cys via peptide bond to the γ-carboxylate of glutamate, which is the rate-limiting step in GSH synthesis (23). Oxidative stress upregulates two subunits of γ-GCS in cells of the skin: the modifier subunit (Gclm) in melanocytes and the catalytic subunit (Gclc) in keratinocytes (24). Although GSH is found in the plasma and extracellular space, it cannot cross cell membranes, and thus must first be hydrolyzed by γ-glutamyl-transferase in order to release Cys (25), which is transported into cells in its reduced or oxidized forms via the ASCT and Xc- transporters, respectively (Fig. 1). The light subunit of Xc- is encoded by Slc7a11 and ASCT is encoded by Slc1a4.
Fig. 1.
NAC metabolism and biomarkers of oxidative stress. NAC is metabolized to cysteine (Cys), which is a glutathione (GSH) precursor. GSH cannot cross cell membranes, but is transformed into Cys and cystine (Cys2) by γ-glutamyl transpeptidases (GGT). Cys and Cys2 enter cells via the amino acid transporters ASCT (Slc1A4) and Xc- (Slc7A11), respectively. Intracellularly, Cys can be reduced by reductases such as TR-1 (Txnrd1) and converted to GSH by γ-glutamylcysteine synthase, the modifier subunit of which is encoded by Gclm and upregulated in UV-irradiated melanocytes and nevi. Reactive oxygen species (ROS) induced by ultraviolet (UV) radiation can lead to formation of 8-oxoguanine (8-OG) and cyclobutane dimers (CPD, see Discussion) in DNA, and modifies the expression of genes such as Txnrd1, Slc1A4, Slc7A11 and Gclm (which encode the proteins TR-1, ASCT, Xc-, and Gclm).
Topically-applied NAC has been used to block UV-induced ROS signaling in human skin (26). We have been investigating its systemic use for the purpose of reducing UV-induced oxidative stress in nevi. Previously, we completed a phase I trial with NAC in 72 patients at increased risk for melanoma (27). We found that an oral dose of 1200 mg was safe and well-tolerated, and that nevi could be treated ex vivo to evaluate NAC-mediated changes in biomarkers of UV-induced oxidative stress. Here we present the results of a phase II randomized placebo-controlled trial in 100 patients, designed to test whether oral NAC could protect nevi against UV-induced oxidative stress in vivo.
Materials and Methods
Patients
This study was approved by the Institutional Review Board (IRB #50308) of the University of Utah. Patients were recruited from the pigmented lesion clinic at the Huntsman Cancer Institute, in which patients with history of numerous or atypical nevi, and/or personal or family history of melanoma are regularly monitored. Patients that were under age 18, critically ill or mentally handicapped, prisoners, pregnant or breast-feeding, non-English speaking, or having history of asthma or allergic reaction to NAC, were excluded. All patients signed a consent form to participate. Patients were not charged for nevus removal or histological examination, and each was compensated $150 following their participation in the pilot study and $200 following participation in the clinical trial or post-trial study.
MC1R testing
Determination of MC1R germline status was performed under a separate IRB protocol (#73694). Saliva samples were collected using an Oragene Discover kit (DNA Genotek, Ottawa, Canada), and genomic DNA was isolated by addition of prepIT-L2P solution (DNA Genotek) followed by ethanol precipitation according to the manufacturer’s instructions. The coding region of the single exon at the MC1R locus was amplified by PCR using primers 5’-GCACCATGAACTAAGCAGGA-3’ and 5’-GGACCAGGGAGGTAAGGAACT-5’, and the presence of mutations was determined by Sanger DNA sequencing. Subjects were categorized as “low-risk” if no mutations or the polymorphisms V60L or V92M were detected, and “high-risk” if heterozygous or homozygous for the following mutations (associated with MC1R loss of function or increased melanoma risk): D84E, R142H, R151C, I155T, R160W, R163Q, D294H, as described elsewhere (28,29).
Nevus tissues
Up to two nevi (≥ 5 mm in diameter to provide sufficient tissue for analysis) on each subject that were not clinically suspicious for melanoma were selected. Following injection of sodium bicarbonate-buffered 1% lidocaine containing 1:100,000 epinephrine, nevi were removed by shave/saucerization technique using a DermaBlade® (Medline Industries, Mundelein, IL), and then a representative 1 mm slice was placed in 10% formalin for paraffin embedding and sectioning. The remaining nevus fragments were grossly macrodissected from normal surrounding skin and immediately placed on ice for 8-OG analysis or frozen at −80 °C in 700 µL QIAzol regent (Qiagen, Valencia, CA) for RNA analysis. A hematoxylin/eosin-stained section was later reviewed by a dermatopathologist (S.R.F.) to confirm the lesion was a nevus and not melanoma. Additional unstained sections were prepared for immunohistochemistry.
Solar-simulated irradiation of nevi
A 16S-300-003 SolarLight device was used as described previously (30). Additional information on the device and minimal erythemal dose (MED) testing is provided in Supplementary Information. Nevi were irradiated at doses of 0.5–12 MED in the pilot study, or 2 (first 50 patients in the trial) or 1 MED (last 50 patients in the trial and 10 patients in the post-trial study).
Measurement of GSH
Quantification of GSH in nevus fragments was determined by reverse-phase chromatography as described previously (27).
Determination of 8-OG in nevus melanocytes
Nevus fragments were diced with a razor blade, transferred to a 15 mL conical tube containing 1 mL of 0.25% Trypsin/EDTA (ThermoFisher Scientific, Waltham, MA), and after vortexing were incubated for 45 min in a 37°C water bath. Next, 2 mL of Medium 254 (ThermoFisher Scientific) containing 10% FBS and 1% pen-strep-glutamine was added. After vortexing, cells were centrifuged at 4000 rpm for 5 min at room temperature, and then resuspended in 1 mL PBS. Cells were separated from debris, transferred to a new 1.5 mL tube, and recentrifuged. The cell pellet was resuspended in 1 mL PBS containing 4% paraformaldehyde at room temperature. After 15 min, cells were centrifuged at 1000 rpm for 5 min and washed once with PBS. Cells were incubated in 1 mL PBS containing 0.2% TritonX-100 (Roche Life Science, Indianapolis, IN) for 5 min, washed in PBS, and then resuspended in 0.1 mL PBS containing 1:50 dilution of Melan A (mouse IgG, MA1-26245, ThermoFisher Scientific) and incubated overnight at 4°C. Cells were centrifuged, washed in PBS, then stained for 8-OG using the OxyDNA Fluorometric Assay kit (Calbiochem, Billerica, MA) according to the manufacturer’s instructions. Briefly, cells were incubated in 0.2 mL of the provided washing solution containing a 1:10 dilution of 8-OG-FITC-conjugate and a 1:200 dilution of secondary antibody for Melan-A (goat anti-mouse IgG, A11032 Alexa Fluor 594, Molecular Probes, Eugene, OR) in the dark for 1 h at 37°C. Cells were pelleted, washed in PBS, then resuspended in 30 µL PBS and added to a glass slide with one drop of ProLong Gold anti-fade reagent (P36935, Molecular Probes) containing 4,6-diamidino-2-phenylindole (DAPI) and then coverslipped. After drying, slides were stored under foil at 4°C until examined by fluorescence microscopy.
Thioredoxin reductase (TR) expression in nevi
Nevus sections were deparaffinized in CitriSolv Clearing Agent (ThermoFisher Scientific) then successively washed in 100%, 95%, 80%, and 70% ethanol. After washing in water, slides were placed in antigen retrieval buffer (10mM sodium citrate, pH 6.0, containing 0.05% Tween 20) and heated for 20 min in a microwave. After cooling, slides were washed repeatedly in water, and then PBS. Blocking buffer (PBS containing 1% BSA and 0.05% Tween-20) was added to prevent non-specific binding, and drained off after 30 min. Primary antibodies against Melan-A (rabbit IgG NBP1-30151, Novus Biologicals, Littleton, CO) and TR-1 (mouse IgG sc-28321, Santa Cruz Biotechnology, Santa Cruz, CA) were diluted 1:200 and 1:500, respectively, in blocking buffer and added to sections which were incubated at 4° C in a humidified chamber overnight. After washing with PBS containing 0.05% Tween-20, secondary antibodies for Melan-A (goat anti-rabbit IgG, A11012 Alexa Fluor 594, Molecular Probes) and TR-1 (goat anti-mouse IgG A11001 Alexa Fluor 488, Molecular Probes) were diluted 1:200 in PBS containing 1% normal goat serum, 1% BSA, and 0.05% Tween-20 and added to sections at room temperature for 1 h. After washing in PBS containing 0.05% Tween-20, one drop of ProLong Gold (Molecular Probes) containing DAPI was added before coverslipping. Slides were stored under foil at −20 °C until examination by fluorescent microscopy. For quantification of TR-1 expression, stained slides were viewed on a Nikon Eclipse Ti microscope with a 20X objective, and images were captured using an Andor Clara camera with Nikon NIS Elements software (version 4.30), and subsequently analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Gene expression studies
Nevus samples were disrupted in 0.2 mL Qiazol at 4 °C in an eppendorf tube using a plastic pestel, then stored at −80 °C until further processing. RNA was purified using an RNeasy kit (Qiagen) following the manufacturer’s instructions. Eluted RNA was quantified on a Synergy H1 microplate reader (BioTek, Winooski, VT) using accompanying Gen5 2.05 software. Further details, including the primer sequences used, is provided in Supplementary Information. A Rotor-Gene Q thermocycler (ThermoFisher Scientific) was used and the data collected using the accompanying software, version 2.0.2. The fold change calculation used the ΔΔCt method with the RPLP0 reaction serving as the endogenous normalization control for each sample as described (31).
NAC administration
To mask its salty taste, N-acetylcysteine solution (NAC, 200 mg per mL solution, American Regent, Shirley, NY) was diluted approximately 1:4 in Campbell’s tomato juice immediately prior to use. Placebo was similarly prepared, substituting normal saline (0.9% sodium chloride) for NAC. The dilutions were prepared behind a curtain by a staff member from our clinical trials office, with a vial of NAC left open on the counter (so the odor of the drug was present in the room), to insure that both the subjects and the investigators were blinded as to which treatment was administered.
Statistics
A biostatistician (K.M.B.) constructed the patient randomization scheme, and performed all statistical analyses using “R” software, version 2.8.0 (Vienna, Austria). P values ≤ 0.05 were considered statistically significant. Patients were randomized to receive NAC or placebo, and stratified to insure equal proportions of MC1R low- and high-risk genotypes in each arm. Robust analysis of covariance methods (R function “lmrob”) was used to analyze relationships between individual categorical variables and change in log MED, with categorical variable and log pre-treatment MED as predictors and log post-treatment MED as response. A Wilcoxon test was used to assess significance of differences in the median percent nevus melanocytes with 8-OG expression in the UV-irradiated nevus compared to that in the control (unirradiated) nevus. Secondary analyses were performed comparing the change in UV-induced 8-OG staining of nevi between treatment groups and the modification of treatment effect on the change in 8-OG by high-risk MC1R mutations. The R function lmrob was used to analyze effect ratios (log TR-1 expression in nevus melanocytes in the UV-irradiated nevus compared to that in the control unirradiated nevus), with categorical variable and pre-treatment TR-1 staining as predictors and post-treatment TR-1 staining as response. For RNA expression studies, the R function lmrob was used to examine the relationship between individual categorical variables and the change in ΔCT, with categorical variable and ΔCT in the un-irradiated nevus as predictors and ΔCT in the irradiated nevus as response. The expression ratio was estimated as 2−ΔCT.
Results
Pilot study of UV-induced markers of oxidative stress in vivo
Forty-one subjects were initially recruited for a pilot study (without drug) in which several parameters for UV-induced modulation of biomarkers of oxidative stress in nevi could be evaluated. We used solar-simulated radiation (SSR) to irradiate nevi in vivo. Irradiated (but not unirradiated) nevi consistently exhibited erythema clinically, and dyskeratotic cells histologically, 24–48 h following SSR ranging from 0.5–12 MED (Supplementary Fig. S1). In our prior ex vivo studies, nevus GSH levels was a primary endpoint since NAC would be predicted to support GSH biosynthesis. Previously, we observed GSH depletion in UV-treated nevi (compared to unirradiated nevi on the same subjects) at 24 and 48 h following UV exposure (27). For 27 subjects in this pilot study, one nevus was treated with SSR at doses ranging from 2–12 MED and then removed 2–48 h later. A control (unirradiated) nevus was also removed from each subject at the same time, and GSH levels were quantitated in each nevus. We found that GSH levels in SSR-irradiated nevi were consistently higher or the same as in control unirradiated nevi (Supplementary Fig. S2). Thus in contrast to our prior findings ex vivo, we did not observe GSH depletion as a marker of UV-induced oxidative stress in nevi in vivo.
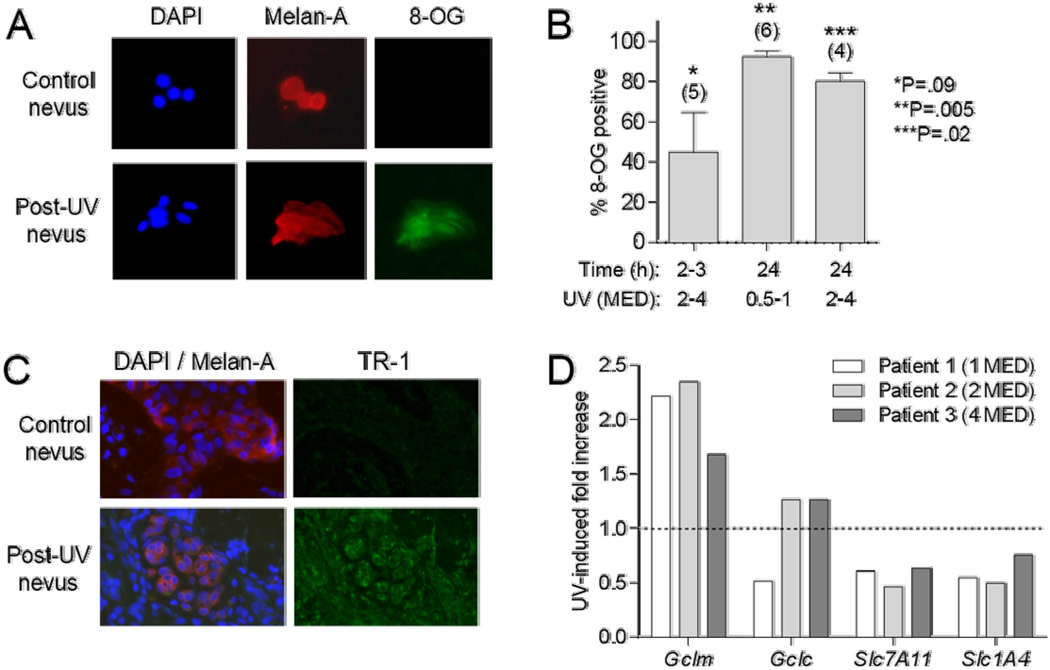
In 15 subjects, we assessed formation of 8-OG in nevi 2–24 h following treatment with SSR (0.5–4 MED). Nevus cells were dissociated and subjected to multi-color immunofluorescence staining (Fig. 2A). Positive staining for 8-OG in UV-treated nevi increased from approximately 45% of the cells at 2 h to 80–100% at 24 h after irradiation (Fig. 2B). In matched unirradiated nevi from the same patients, 8-OG positivity ranged from 0–70% and averaged around 40% (not shown). While this level of detection may represent background staining or actual oxidative stress associated with nevus manipulation and the staining procedure, we consistently detected a higher percentage of cells expressing 8-OG in each UV-treated nevus compared to the matched unirradiated nevus. Across a range of UV doses, there were significantly (P=.005, .02) more 8-OG positive cells detected from irradiated vs. unirradiated nevi at the 24-h time point (Fig. 2B). We did not measure ROS in the nevi, but have previously reported that 8-OG expression correlates with ROS in UV-irradiated human melanocytes (32). In 10 subjects, we assessed TR-1 expression in fixed sections of unirradiated nevi or nevi 6h or 24 h following SSR treatment (0.5–4 MED) over a 15-fold concentration range of primary antibody. Staining intensity increased with antibody concentration and dose of SSR, and uniform melanocyte TR-1 staining was consistently observed in SSR-treated compared to unirradiated nevi at SSR doses of ≥1 MED at 24 h (Fig. 2C). Thus 8-OG and TR-1 proved to be reliable immunohistochemical markers of UV-induced oxidative stress in nevi in vivo.
Fig. 2.
UV-induced markers of oxidative stress. A, Representative staining of dissociated nevus cells for DAPI, Melan-A, or 8-OG. B, Shown are percent nevus melanocytes positive for 8-OG, at indicated time and UV dose ranges. Error bars indicate SEM. Numbers of patients in each group indicated in parentheses. P values for comparisons of values for irradiated vs. unirradiated tissues at each condition determined by paired t tests. C, Representative staining of nevus sections for DAPI, Melan-A, and TR-1. D, Expression of Gclm, Gclc, Slc7A11, and Slc1A4 genes from RNA isolated from control and UV-irradiated nevi 24h after SSR exposure (1–4 MED) in three subjects. Data expressed as ratio of normalized signals from UV-treated to control nevi.
In separate studies using human melanocytes that were UV-treated in vitro, we evaluated RNA expression of anti-oxidant-related genes and found several that were consistently modulated by UV exposure. Genes relevant to NAC metabolism included subunits of the γ-GCS and the Cys transporters Slc1a4 and Slc7a11. In 3 patients, we assessed expression levels for these four genes in SSR-treated nevi (1–4 MED) at 24 h and in matched unirradiated nevi. As shown in Fig. 2D, Gclm (but not Gclc) was consistently upregulated by UV treatment, and Slc1a4 and Slc7a11 were down-regulated. Thus we used these three genes going forward as relevant biomarkers of response to SSR in nevi in vivo.
Phase II trial
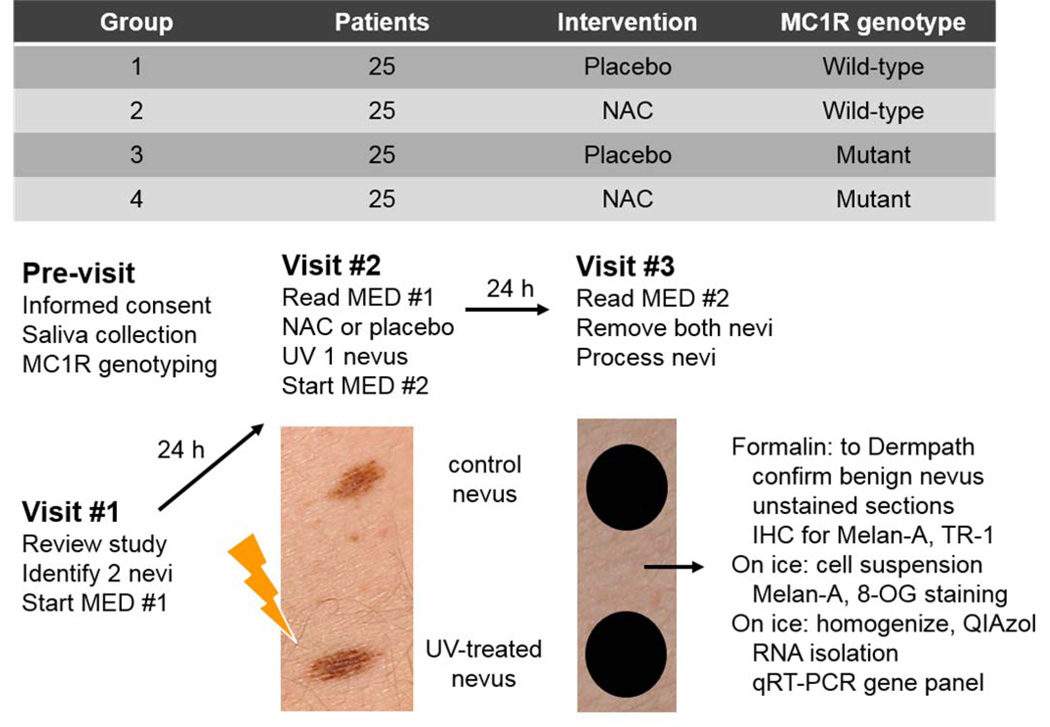
One hundred subjects were recruited for a randomized placebo-controlled trial to examine whether NAC could modulate these markers in nevi in the setting of acute UV exposure. The trial design is depicted in Fig. 3, and Supplementary Table I details the characteristics of the subjects, who were randomized to receive NAC or placebo. For all subjects, germline MC1R status was evaluated by DNA sequencing and this was factored into the randomization scheme, so that subjects with low and high risk MC1R alleles would be equally distributed into the drug and placebo arms. After determination of the MED, subjects were given NAC or placebo. Immediately thereafter, one nevus was SSR-treated (at a dose based on each subject’s MED) and the next day (approximately 24 h later), both the irradiated nevus and an unirradiated nevus were removed for analysis. Histologic examination of a representative fragment from each lesion revealed that all were benign neoplasms.
Fig. 3.
Subject randomization and clinical trial design. Individuals were randomized to one of four groups such that half were scheduled to receive drug and half to receive placebo. Based on sequencing of the MC1R gene, approximately half the subjects were wild-type (WT) and half had at least one germline high-risk allele (mutant). At the first visit, minimal erythemal dose (MED) testing was initiated. After 24h, the MED was determined and either 1200 mg N-acetylcysteine (NAC) or placebo was administered. One nevus was UV-irradiated (1–2 MED), and a second round of MED testing was initiated. After 24h, the post-intervention MED was determined, and the UV-irradiated nevus and a control unirradiated nevus were removed. Each nevus was fragmented for histologic, immunohistochemical, and molecular analyses.
The MED results are presented in Table I. While the ratio of post-treatment MED to pre-treatment MED was 5% lower in the NAC vs. placebo group, this difference was not statistically significant (P=0.068). We also did not observe a difference in the ratio of MEDs between subjects with low-risk and high-risk MC1R alleles. We tested for a potential interaction between treatment and MC1R status variables, but did not find one (P=0.745). Analysis of 8-OG and TR-1 staining in UV-irradiated nevi is summarized in Table II. All SSR-treated nevi exhibited a high percentage of melanocyte 8-OG staining (~95%) compared to unirradiated nevi (~33%), which was statistically significant (P<0.001 for placebo group). However, there was not a significant difference between samples from patients given NAC or placebo (P=0.65). Similarly, we did not observe a difference in 8-OG staining between subjects with low-risk and high-risk MC1R alleles (P=0.48). We did not find a significant interaction between treatment and MC1R status variables (not shown). Melanocyte staining for TR-1 was increased in SSR-treated compared to unirradiated nevi (P= 0.003 for placebo group), but we did not observe a significant difference between samples from patients given NAC or placebo (P=0.43). Similarly, we did not observe a difference in TR-1 staining between subjects with low-risk and high-risk MC1R alleles (P=0.54). We did not find a significant interaction between treatment and MC1R status variables (not shown). Finally, we examined expression levels of three genes from RNA isolated from the nevi, which are summarized in Table III. Levels of Gclm were increased (ΔCT decreased) in irradiated nevi compared to unirradiated nevi (P<0.001 for placebo group), but we did not observe a significant difference between samples from patients given NAC or placebo (P=0.76). Similarly, we did not observe a difference in Gclm expression changes between subjects with low-risk and high-risk MC1R alleles (P=0.52). Levels of Slc1A4 and Slc7A11 were decreased in irradiated nevi compared to unirradiated nevi (not significant, P=0.12, 0.62 for placebo groups respectively), and we did not observe a significant difference between samples from patients given NAC or placebo (P=0.63, 0.73 respectively). Similarly, we did not observe a difference in expression changes of Slc1A4 or Slc7A11 between subjects with low-risk and high-risk MC1R alleles (P=0.27, 0.40 respectively). We did not find a significant interaction between treatment and MC1R status variables for any of these three genes (not shown). Thus UV-induced modulation of markers of oxidative stress in nevi was not significantly different in subjects receiving NAC compared to those receiving placebo.
Table I.
Analysis of minimal erythemal dose (MED)
| Univariate analysis | ||
|---|---|---|
| Variable | Patients | Effect ratio* (95% CI), P value |
| Treatment | ||
| Placebo | 50 (50%) | 1.00 |
| Drug | 50 (50%) | 0.95 (0.91–1.00), 0.068 |
| MC1R status | ||
| Low-risk | 50 (50%) | 1.00 |
| High-risk | 50 (50%) | 1.00 (0.95–1.05), 0.941 |
Effect ratio is the ratio of post-treatment MED to pre-treatment MED.
Additional analyses of age, gender, height, weight, hair color, and eye color as covariates did not reveal any significant predictors (not shown). We did not find a significant interaction between treatment and MC1R status variables (P=0.745)
Table II.
Immunohistologic staining for markers of UV-induced oxidative stress
| Variable | Patients* | Percent 8-OG (mean, ±SD) | Univariate analysis | |
|---|---|---|---|---|
| Control | UV-irradiated | P value# | ||
| Treatment | ||||
| Placebo | 49 (51%) | 33.1 ±24.2 | 96.2 ±7.5∥ | |
| Drug | 48 (49%) | 33.6 ±25.1 | 94.6 ±12.3 | 0.65 |
| MC1R status | ||||
| Low-risk | 48 (49%) | 35.9 ± 26.9 | 95.8 ± 11.0 | |
| High-risk | 49 (51%) | 30.8 ± 21.9 | 95.0 ± 9.3 | 0.48 |
| Log (x100) TR-1 (mean, ±SD) | Univariate analysis | |||
| Control | UV-irradiated | Effect ratio┼ (95% CI), P value | ||
| Treatment | ||||
| Placebo | 50 (51%) | 1.05 ±0.76 | 1.26 ±0.70╪ | 1.00 |
| Drug | 49 (49%) | 1.16 ±0.58 | 1.28 ±0.55 | 0.94 (0.81 – 1.10), 0.43 |
| MC1R status | ||||
| Low-risk | 49∥ (49%) | 1.00 | ||
| High-risk | 50 (51%) | 0.95 (0.82 – 1.11), 0.54 | ||
One subject with low-risk MC1R randomized to drug was excluded from both analyses because the lesions removed were seborrheic keratoses and not nevi, and two subjects (one high-risk MC1R randomized to drug and one low-risk MC1R randomized to placebo) were excluded from analysis of 8-OG because there was insufficient tissue.
Wilcoxon test was used to assess significance of differences in the median percent nevus melanocytes with 8-OG expression in the UV-irradiated nevus compared to that in the control (unirradiated) nevus. Additional analyses of 8-OG response with gender, height, weight, hair color, and eye color as covariates did not reveal any significant predictors (not shown).
For placebo group, difference between control and UV-irradiated was significant (P<0.001, Wilcoxon test).
The effect ratio is the ratio of log TR-1 expression in nevus melanocytes in the UV-irradiated nevus compared to that in the control (unirradiated) nevus. Additional analyses of TR-1 response with age, gender, height, weight, hair color, and eye color as covariates did not reveal any significant predictors (not shown).
For placebo group, difference between control and UV-irradiated was significant (P=0.003, Wilcoxon test).
We tested for potential interaction between treatment and MC1R status for both analyses, and did not find significance for either (not shown).
Table III.
Analysis of transcriptional markers of UV-induced oxidative stress
| Univariate analysis | ||||
|---|---|---|---|---|
| Variable | Patients* | ΔCT#Gclm (mean, ±SD) | Analysis of Covariance | |
| Control | UV-irradiated | Expression ratio∥ (95% CI), P value | ||
| Treatment | ||||
| Placebo | 50 (51%) | 9.74 ±0.69 | 9.14 ±0.75┼ | 1.00 |
| Drug | 49 (49%) | 9.42 ±0.98 | 8.99 ±0.83 | 0.97 (0.82 – 1.16), 0.76 |
| MC1R status | ||||
| Low-risk | 49 (49%) | 1.00 | ||
| High-risk | 50 (51%) | 1.07 (0.88 – 1.30), 0.52 | ||
| ΔCT Slc1A4 (mean, ±SD) | ||||
| Control | UV-irradiated | |||
| Treatment | ||||
| Placebo | 50 (51%) | 9.21 ±0.83 | 9.40 ±1.06╪ | 1.00 |
| Drug | 49 (49%) | 9.28 ±0.89 | 9.29 ±0.87 | 1.06 (0.84–1.34), 0.63 |
| MC1R status | ||||
| Low-risk | 49 (49%) | 1.00 | ||
| High-risk | 50 (51%) | 1.04 (0.83–1.31), 0.73 | ||
| ΔCT Slc7A11 (mean, ±SD) | ||||
| Control | UV-irradiated | |||
| Treatment | ||||
| Placebo | 50 (51%) | 8.97 ±1.04 | 9.03 ±0.95╫ | 1.00 |
| Drug | 49 (49%) | 8.62 ±0.96 | 8.73 ±1.13 | 1.16 (0.90 – 1.49), 0.27 |
| MC1R status | ||||
| Low-risk | 49 (49%) | 1.00 | ||
| High-risk | 50 (51%) | 1.11 (0.87–1.43), 0.40 | ||
One subject with low-risk MC1R randomized to drug was excluded from the analyses because the lesions removed were seborrheic keratoses and not nevi.
ΔCT is the difference in CT between the gene of interest and RPLP0.
Analysis of covariance was used to examine the relationship between individual categorical variables and the change in ΔCT, with categorical variable and ΔCT in the un-irradiated nevus as predictors and ΔCT in the irradiated nevus as response. Additional analyses of age, gender, height, weight, hair color, and eye color as covariates did not reveal any significant predictors (not shown). The expression ratio is estimated as 2−ΔCT.
For placebo group, difference between control and UV-irradiated was significant (P<0.001, Wilcoxon test).
For placebo group, difference between control and UV-irradiated was not significant (P=0.012, Wilcoxon test).
For placebo group, difference between control and UV-irradiated was not significant (P=0.062, Wilcoxon test).
We tested for potential interaction between treatment and MC1R status for each analysis, and did not find significance for any of them (not shown).
Post-trial study
We considered the possibility that NAC (or its metabolites) needed to be present in nevi at the time of UV exposure to have a greater effect. In our prior study, we detected metabolites of NAC in nevi 3 h after ingestion (27). We did not attempt to detect NAC metabolites such as Cys in the nevus samples in this study which were all obtained 24 h following drug ingestion since we had previously found that these metabolites were undetectable in nevi after 6 h (27). We recruited 10 subjects for a post-trial open label study in which all received NAC and nevi were SSR-treated (1 MED) 3 h after NAC ingestion. Half of the subjects (5/10) had a high-risk MC1R allele. Both unirradiated and irradiated nevi were removed approximately 24 h after SSR exposure, and then analyzed for 8-OG and TR-1 staining, and expression of the Gclm, Slc1A4, and Slc7A11 genes. For these analyses, SSR responses for these 10 patients who received drug were compared to the (49–50) patients who received placebo in the randomized trial above. The data are presented in Supplementary Table II. The SSR-induced responses in nevi were not significantly different between the patients who received NAC 3 h before SSR treatment and those who received placebo.
Discussion
We have now examined the chemoprotective effects of NAC on UV-induced oxidative stress in three studies, ranging from pre-clinical to human trials. Our prior and current findings are summarized in Table IV. First, we showed that NAC reduced intracellular ROS and 8-OG in UV-treated melanocytes and that oral delivery of NAC restored GSH levels and blocked 8-OG formation in UV-treated skin (18). In melanoma-prone mice (33), oral NAC significantly delayed melanoma tumor onset (18). Second, we completed a Phase Ib trial in which we established the safety of a single 1200 mg oral NAC in human subjects and showed that NAC could protect against UV-induced depletion of GSH in nevi irradiated ex vivo (27). In the pilot phase of the third study described here, however, we did not observe GSH depletion in nevi irradiated in vivo. This difference may indicate that we do not fully understand the dynamics of GSH metabolism in vivo. In the subsequent phase II trial reported here, we examined the effects of NAC on endpoints that were modulated by UV in vivo, namely 8-OG formation, TR-1 expression, and mRNA levels of Gclm, Slc1A4, and Slc7A11.
Table IV.
Summary of prior and current studies
| Conditions | UV | Study type | Biomarker | Results | Reference |
|---|---|---|---|---|---|
| Mouse | FS20T12 | Controlled | Free thiols | ↓ after UV | Cotter (18) |
| model | Free thiols | ↓ UV-depletion after NAC | |||
| 8-OG | ↑ after UV | ||||
| 8-OG | ↓ UV-depletion after NAC | ||||
| Melanoma | NAC delays tumor onset | ||||
| Ex-vivo | FS20T12 | Open label, | GSH | ↑ after UV | Goodson (27) |
| nevi | Phase Ib | ROS | ↓ after UV | ||
| GSH | ↑ 3 h after NAC | ||||
| Cys | ↑ 3 h after NAC | ||||
| GSH | ↓ UV-depletion after NAC (~ half of subjects) |
||||
| In-vivo | SSR* | Pilot study | GSH | not ↓ after UV | Present study |
| nevi | (no drug) | 8-OG | ↑ after UV | ||
| TR-1 | ↑ after UV | ||||
| Gclm | ↑ after UV | ||||
| Slc1A4 | ↓ after UV | ||||
| Slc7A11 | ↓ after UV | ||||
| In-vivo | SSR | Phase II, | 8-OG | ↑ after UV, no NAC effect | Present study |
| nevi | blinded, | TR-1 | ↑ after UV, no NAC effect | ||
| controlled# | Gclm | ↑ after UV, no NAC effect | |||
| Slc1A4 | ↓ after UV, no NAC effect | ||||
| Slc7A11 | ↓ after UV, no NAC effect | ||||
| In-vivo | SSR | Open label, | 8-OG | ↑ after UV, no NAC effect | Present study |
| nevi | post-trial | TR-1 | ↑ after UV, no NAC effect | ||
| study∥ | Gclm | ↑ after UV, no NAC effect | |||
| Slc1A4 | ↓ after UV, no NAC effect | ||||
| Slc7A11 | ↓ after UV, no NAC effect |
SSR, solar-simulated radiation
NAC given immediately prior to UV treatment
NAC given 3 h prior to UV treatment
Several differences in the experimental designs of our Phase I and II trials are important to consider. First is the elapsed time between NAC administration and UV treatment, which was 3 h in the Phase I study since we could detect Cys in nevi 3 h after NAC ingestion (27). In the Phase II trial, we irradiated nevi immediately after NAC administration, largely for practical considerations. We believed that this small change would be inconsequential given the evidence from both in vitro and in vivo models that elevated ROS levels and DNA damage appear almost immediately after UV exposure and last for at least 3–5 h (16,18,32,34). In fact, a recent study described production of cyclobutane dimers in melanocytes 3 h after UV exposure (so-called dark CPDs) that were suppressed by NAC (34). However, when we failed to see an effect of NAC on any of our endpoints, we did consider the possibility that Cys itself may act directly to quench ROS, instead of primarily serving as a source for the re-synthesis of GSH. Under this scenario, the absence of supplemental Cys in the tissues at time of irradiation might decrease the therapeutic effect of NAC. Thus we did perform a small (post-trial) study in which nevi were SSR-treated 3 h after NAC administration, but unfortunately did not see protection by the drug. Therefore we do not believe that timing of NAC ingestion relative to UV treatment was the major (or only) reason that we failed to see any effect of the drug on biomarkers of UV-induced oxidative stress and damage.
A second consideration is the UV light sources that were used. Although the FS20T12 bulbs (27) used for the Phase I trial and the SSR device (30) used here cause the same spectrum of mutations and have a similar effect on melanoma development in transgenic mice (35,36), they differ significantly in the relative proportion of UVA and UVB (FS20T12: 20% UVA/80% UVB; SolarLight: 92% UVA/8% UVB) emitted. These UV sources may have differential effects on human skin, including modulation of the innate and cellular immune systems and UV-induced production of nitric oxide and ROS (37), which could potentially affect the endpoints that we evaluated as well as the effects of NAC.
A third consideration is the conditions under which the nevi were irradiated. In the Phase I trial, nevi were irradiated ex vivo and then incubated at 37 °C for 24–48 h in room air supplemented with 5% CO2. In the Phase II trial, nevi were irradiated in vivo and removed 24 h later for analysis. The ex vivo nevi were likely significantly hyperoxic compared to those treated in vivo, as the pO2 is approximately 3–4 times greater in an incubator than in vivo (38). This situation is analogous to that encountered during ischemia and reperfusion, where the redox buffering capacity of the tissues are significantly altered by fluctuations in oxygen levels (39). It is exactly this GSH-dependent redox capacity that we are seeking to support by supplementing the tissue with NAC, therefore it is not unreasonable to speculate that changes in the conditions under which the tissues were treated could significantly influence the outcomes of our two studies.
In addition to UV exposure, mutations in MC1R (28) and CDKN2A (32) are also implicated in elevated oxidative stress in melanocytes. These and other genetic factors could potentially influence the acute responses of individual nevi to UV irradiation and the capacity for NAC-mediated protection. In both of our studies, each patient served as their own control because one nevus was removed prior to NAC administration (Phase I study) or was not irradiated (Phase II study). The ex vivo design of the Phase I study allowed us to divide nevi in half and analyze both irradiated and unirradiated nevi, before and after NAC. Efforts to control for inter-subject biological variation in this Phase II study included incorporation of MED determination so that we could administer a biologically equivalent dose of SSR to each participant (while UV dosing was not MED-based in the Phase I study). In the Phase I trial we did see a trend towards greater protection with NAC in subjects with high-risk MC1R mutations (40), and thus took this factor into account as we ensured that equal numbers of high- and low-risk mutation carriers were in each arm of the Phase II study.
Although animal studies from our group (18) and others (41) suggest potential utility of NAC in skin cancer prevention, there are reasons to be cautious about the chronic use of antioxidants in this context. Selenium, an antioxidant micronutrient that can support the metabolism of ROS via the selenoproteins thioredoxin reductase and glutathione peroxidase (42), has actually been found to increase risk for non-melanoma skin cancer in high-risk populations (43). Using mice transgenic for hepatocyte growth factor, we previously reported that while a single topical treatment of selenomethionine (SeMet) to neonatal mice 24 h prior to UV-irradiation delayed melanoma formation, continued application of SeMet to the skin of these mice promoted the growth of early-stage tumors (44). Recent studies in other mouse models have raised similar concerns for NAC. Daily administration of NAC increased metastases in a BRAFV600E-driven transgenic model (45) and in patient-derived melanoma xenografts (46). Thus although antioxidants have the potential to lower melanoma risk when utilized at the initiation stage in a primary prevention setting, providing antioxidants to tissue harboring initiated cells or early-stage tumors may accelerate tumor growth and/or metastasis. This would be of particular concern in clinical prevention trials based on chronic antioxidant use where study participants might be melanoma survivors harboring subclinical metastases or early stage tumors at the time of enrollment.
Nevertheless, we believe that administration of antioxidants in the acute setting (i.e. during periods of UV exposure) has the potential to decrease oxidative stress in epidermal tissues while avoiding the potential risks of chronic antioxidant administration. It is possible that our inability to detect any effect of NAC on biomarkers of oxidative stress and damage was related to difficulties inherent in attempting to evaluate the dynamics of these systems in tissues by analyses that must be made at a single time point. It was not practical for participants to undergo repeated nevus biopsies in order to detect changes in biomarkers as a function of time. To overcome this difficulty, we are working to develop non-invasive methods that can be used to make repeated measurements of the effects of UV-induced oxidative stress that will allow us to follow changes in redox status as a function of time. For example, it is now possible to measure antioxidant status in tissues using fluorescence lifetime imaging microscopy of NADH and NADPH (47).
Supplementary Material
Acknowledgments
We acknowledge the assistance of Darren Walker and Andrew Grandemange from our Clinical Trial Office, supported by P30 CA042014 awarded to Huntsman Cancer Institute. This work was also supported by the Bioanalytical and Pharmacokinetics shared resources, supported by P30 CA 069533 awarded to Oregon Health & Science University.
Grant support
P. Cassidy received financial support from the Department of Dermatology at the Oregon Health & Science University. D. Grossman received financial support from the Department of Dermatology and the Huntsman Cancer Foundation at the University of Utah. This work was supported by NIH grant R01 CA166710 to D. Grossman and P. Cassidy.
Abbreviations
- 8-OG
8-oxoguanine
- Cys
cysteine
- GCLM
γ-glutamylcysteine synthase subunit M
- γ-GCS
γ-glutamylcysteine synthase
- GSH
glutathione
- MC1R
melanocortin-1 receptor
- MED
minimal erythemal dose
- NAC
N-acetylcysteine
- ROS
reactive oxygen species
- SSR
solar-simulated radiation
- TR
thioredoxin reductase
- UV
ultraviolet
Footnotes
Conflicts of interest: None declared.
ClinicalTrials.gov number NCT01612221.
References
- 1.Whiteman DC, Green AC, Olsen CM. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.01.035. in press. [DOI] [PubMed] [Google Scholar]
- 2.Lau PK, Ascierto PA, McArthur G. Melanoma: the intersection of molecular targeted therapy and immune checkpoint inhibition. Curr Opin Immunol. 2016;39:30–38. doi: 10.1016/j.coi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Guy GP, Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC, et al. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64:591–596. [PMC free article] [PubMed] [Google Scholar]
- 4.Haenssle HA, Mograby N, Ngassa A, Buhl T, Emmert S, Schon MP, et al. Association of Patient Risk Factors and Frequency of Nevus-Associated Cutaneous Melanomas. JAMA Dermatol. 2016;152:291–298. doi: 10.1001/jamadermatol.2015.3775. [DOI] [PubMed] [Google Scholar]
- 5.Kelly JW, Rivers JK, MacLennan R, Harrison S, Lewis AE, Tate BJ. Sunlight: a major factor associated with the development of melanocytic nevi in Australian schoolchildren. J Am Acad Dermatol. 1994;30:40–48. doi: 10.1016/s0190-9622(94)70005-2. [DOI] [PubMed] [Google Scholar]
- 6.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 7.Lazovich D, Isaksson Vogel R, Weinstock MA, Nelson HH, Ahmed RL, Berwick M. Association Between Indoor Tanning and Melanoma in Younger Men and Women. JAMA Dermatol. 2016;152:268–275. doi: 10.1001/jamadermatol.2015.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shellenberger R, Nabhan M, Kakaraparthi S. Melanoma screening: A plan for improving early detection. Ann Med. 2016;48:142–148. doi: 10.3109/07853890.2016.1145795. [DOI] [PubMed] [Google Scholar]
- 9.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- 10.Herrling T, Jung K, Fuchs J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:840–845. doi: 10.1016/j.saa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Vile GF, Tyrrell RM. UVA radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen. Free Radic Biol Med. 1995;18:721–730. doi: 10.1016/0891-5849(94)00192-m. [DOI] [PubMed] [Google Scholar]
- 12.Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature. 2000;403:859–866. doi: 10.1038/35002510. [DOI] [PubMed] [Google Scholar]
- 13.Korge P, Calmettes G, Weiss JN. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim Biophys Acta. 2015;1847:514–525. doi: 10.1016/j.bbabio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyskens FL, Jr, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14:148–154. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 15.Fried L, Arbiser JL. The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res. 2008;21:117–122. doi: 10.1111/j.1755-148X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 16.Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 17.Wendt J, Rauscher S, Burgstaller-Muehlbacher S, Fae I, Fischer G, Pehamberger H, et al. Human Determinants and the Role of Melanocortin-1 Receptor Variants in Melanoma Risk Independent of UV Radiation Exposure. JAMA Dermatol. 2016 doi: 10.1001/jamadermatol.2016.0050. [DOI] [PubMed] [Google Scholar]
- 18.Cotter MA, Thomas J, Cassidy P, Robinette K, Jenkins N, Florell SR, et al. N-acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet-induced melanoma in mice. Clin Cancer Res. 2007;13:5952–5958. doi: 10.1158/1078-0432.CCR-07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. http://www.accessdata.fda.gov/scripts/cder/drugsatfda.
- 20.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 21.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 22. https://clinicaltrials.gov.
- 23.Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Marrot L, Jones C, Perez P, Meunier JR. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008;21:79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Forman HJ. Redox regulation of gamma-glutamyl transpeptidase. Am J Respir Cell Mol Biol. 2009;41:509–515. doi: 10.1165/rcmb.2009-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S, Chung JH, Lee JH, Fisher GJ, Wan YS, Duell EA, et al. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol. 2003;120:835–841. doi: 10.1046/j.1523-1747.2003.12122.x. [DOI] [PubMed] [Google Scholar]
- 27.Goodson AG, Cotter MA, Cassidy P, Wade M, Florell SR, Liu T, et al. Use of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress: towards a novel paradigm for melanoma chemoprevention. Clin Cancer Res. 2009;15:7434–7440. doi: 10.1158/1078-0432.CCR-09-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadekaro AL, Leachman S, Kavanagh RJ, Swope V, Cassidy P, Supp D, et al. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J. 2010;24:3850–3860. doi: 10.1096/fj.10-158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquali E, Garcia-Borron JC, Fargnoli MC, Gandini S, Maisonneuve P, Bagnardi V, et al. MC1R variants increased the risk of sporadic cutaneous melanoma in darker-pigmented Caucasians: a pooled-analysis from the M-SKIP project. Int J Cancer. 2015;136:618–631. doi: 10.1002/ijc.29018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodson AG, Varedi A, Hull C, Grossman D. A safe and efficient model for ultraviolet radiation-induced herpes simplex labialis. Photodermatol Photoimmunol Photomed. 2015;31:170–172. doi: 10.1111/phpp.12168. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins NC, Liu T, Cassidy P, Leachman SA, Boucher KM, Goodson AG, et al. The p16(INK4A) tumor suppressor regulates cellular oxidative stress. Oncogene. 2011;30:265–274. doi: 10.1038/onc.2010.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas J, Liu T, Cotter MA, Florell SR, Robinette K, Hanks AN, et al. Melanocyte expression of survivin promotes development and metastasis of UV-induced melanoma in HGF-transgenic mice. Cancer Res. 2007;67:5172–5178. doi: 10.1158/0008-5472.CAN-06-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K, et al. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347:842–847. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6376. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- 36.Brash DE. UV signature mutations. Photochem Photobiol. 2015;91:15–26. doi: 10.1111/php.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halliday GM, Byrne SN. An unexpected role: UVA-induced release of nitric oxide from skin may have unexpected health benefits. J Invest Dermatol. 2014;134:1791–1794. doi: 10.1038/jid.2014.33. [DOI] [PubMed] [Google Scholar]
- 38.Spence VA, Walker WF. Tissue oxygen tension in normal and ischaemic human skin. Cardiovasc Res. 1984;18:140–144. doi: 10.1093/cvr/18.3.140. [DOI] [PubMed] [Google Scholar]
- 39.Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, et al. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 40.Cassidy P, Leachman S, Grossman D. N-acetylcysteine for reduction of oxidative stress/damage and prevention of melanoma. In: Watson R, Zibadi S, editors. Bioactive dietary factors and plant extracts in dermatology. New York: Springer; 2013. pp. 341–356. [Google Scholar]
- 41.D’Agostini F, Balansky RM, Camoirano A, De Flora S. Modulation of light-induced skin tumors by N-acetylcysteine and/or ascorbic acid in hairless mice. Carcinogenesis. 2005;26:657–664. doi: 10.1093/carcin/bgi008. [DOI] [PubMed] [Google Scholar]
- 42.Cassidy PB, Honeggar M, Poerschke RL, White K, Florell SR, Andtbacka RH, et al. The role of thioredoxin reductase 1 in melanoma metabolism and metastasis. Pigment Cell Melanoma Res. 2015;28:685–695. doi: 10.1111/pcmr.12398. [DOI] [PubMed] [Google Scholar]
- 43.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, Fischbach LA, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 44.Cassidy PB, Fain HD, Cassidy JP, Jr, Tran SM, Moos PJ, Boucher KM, et al. Selenium for the prevention of cutaneous melanoma. Nutrients. 2013;5:725–749. doi: 10.3390/nu5030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aad3740. 308re8. [DOI] [PubMed] [Google Scholar]
- 46.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blacker TS, Mann ZF, Gale JE, Ziegler M, Bain AJ, Szabadkai G, et al. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun. 2014;5:3936. doi: 10.1038/ncomms4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.