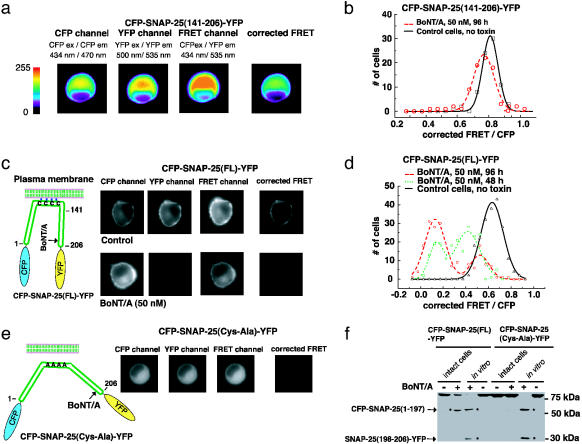
Fig. 2.
Monitoring BoNT/A activity in living cells. (a) Measuring toxin sensor FRET in living cells. CFP-SNAP-25(141–206)-YFP was expressed in wild-type PC12 cells; this protein has a uniform cytosolic distribution. Three images using different filter set (CFP, FRET, and YFP) were taken for each cell sequentially, using exactly the same settings. Images were color coded to reflect the fluorescence intensity in arbitrary units as indicated in the key. The corrected FRET value was calculated by subtracting the cross-talk from both CFP and YFP from the signals collected by using the FRET filter set, as detailed in the Materials and Methods. (b) PC12 cells that express CFP-SNAP-25(141–206)-YFP were used to detect BoNT/A activity. BoNT/A holotoxin was added to the culture medium, and 80 cells were analyzed after 96 h. The corrected FRET signal was normalized to the CFP fluorescence signal and plotted as a histogram with the indicated bins. Control cells, not treated with toxins, were analyzed in parallel. (c) A sensitive toxin biosensor was created by using full-length SNAP-25. (Left) An alternative toxin sensor was built by linking CFP and YFP through full-length SNAP-25 (amino acids 1–206); a schematic representation is shown. Full-length SNAP-25 is anchored to the plasma membrane via the palmitoylation of four cysteine residues. (Right) The CFP-SNAP-25(FL)-YFP sensor was expressed in PC12 cells. BoNT/A holotoxin was added to the culture medium, and the FRET signals of 200 cells were analyzed after 48 and 96 h as described in a. Control cells were not treated with toxins and were analyzed in parallel. Images of representative cells are shown. This sensor yielded significant FRET (upper “corrected FRET”), which was abolished after cells were treated with BoNT/A (96 h, lower “corrected FRET”). Note that one of the cleavage products, the C terminus of SNAP-25 tagged with YFP, was rapidly degraded after toxin cleavage. Thus, the fluorescence signal of YFP was significantly decreased in toxin-treated cells (lower “YFP”). (d) The FRET ratios of cells analyzed in c were plotted as a histogram with indicated bins, as described in b. (e) PC12 cells were transfected with CFP-SNAP-25(Cys-Ala)-YFP (full length SNAP-25 with Cys 85,88,90,92 Ala mutations). This protein was diffusely distributed throughout the cytosol and lacked the strong FRET signal observed for CFP-SNAP-25(FL)-YFP (“corrected FRET” frame, Fig. 5c). (f) PC12 cells were transfected with CFP-SNAP-25(FL)-YFP and CFP-SNAP-25(Cys-Ala)-YFP. Cells were incubated with (+, intact cells) or without (–, intact cells) BoNT/A (50 nM, 72 h), and then harvested and analyzed. In parallel, half of the cell lysates from samples that had not been exposed to BoNT/A were also subsequently incubated with (+, in vitro) or without (–, in vitro) reduced BoNT/A (200 nM) in vitro (30 min, 37°C); these samples served as controls to show the cleavage products that accumulate after the action of the toxin (two cleavage products are indicated by arrows). The same amount of each sample (30-μg cell lysate) was subjected to SDS/PAGE and immunoblot analysis using an anti-GFP antibody, which recognizes both CFP and YFP. Whereas CFP-SNAP-25(FL)-YFP underwent significant cleavage in intact cells, there was no detectable cleavage of CFP-SNAP-25(Cys-Ala)-YFP, indicating that membrane anchoring of SNAP-25 is important for efficient cleavage by BoNT/A in cells. Note that only one cleavage product [CFP-SNAP-25(1–197)] was detected in toxin-treated cells, indicating that the other cleavage product [SNAP-25(198–206)-YFP] was rapidly degraded in cells.