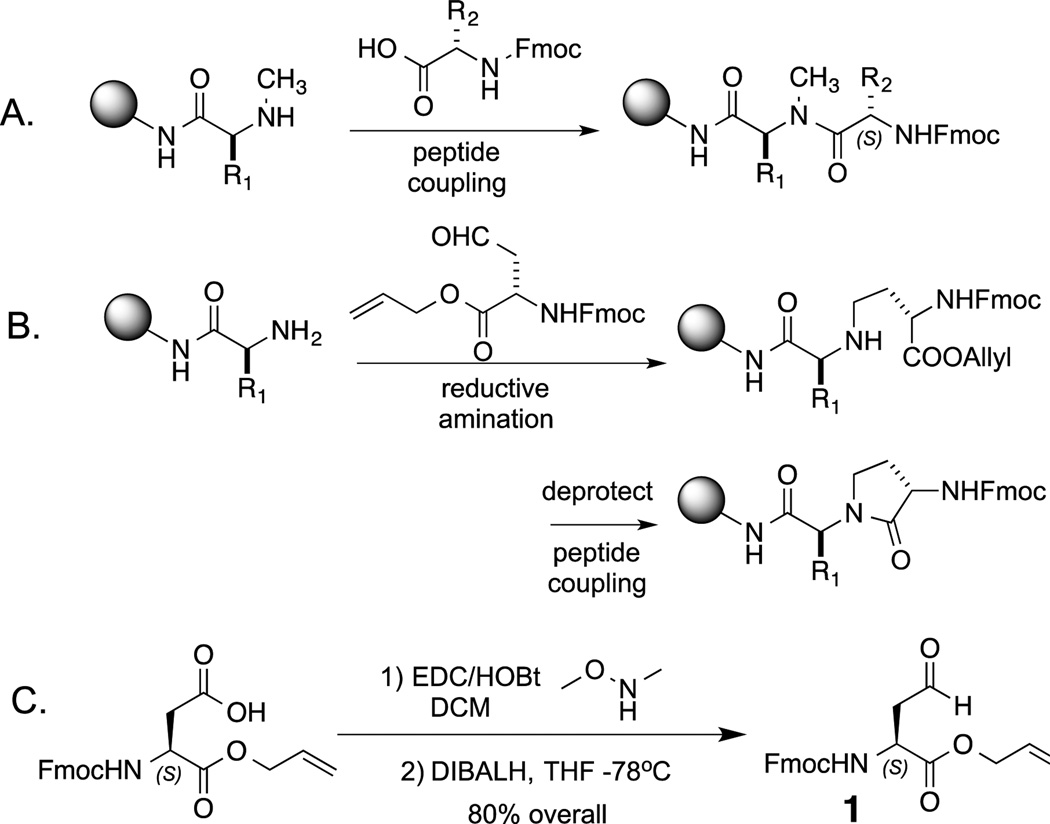
Fig. 1.
A new strategy for morphing peptides into more serum-stable, cell permeable molecules via N-alkylation. A. The traditional insertion of N-methyl amino acids into peptides necessitates a difficult acylation of the secondary amine. B. The CyAla strategy described here renders the acylation of the secondary amine an intramolecular reaction. C. Synthesis of the building block used to insert a CyAla residue into a peptide.