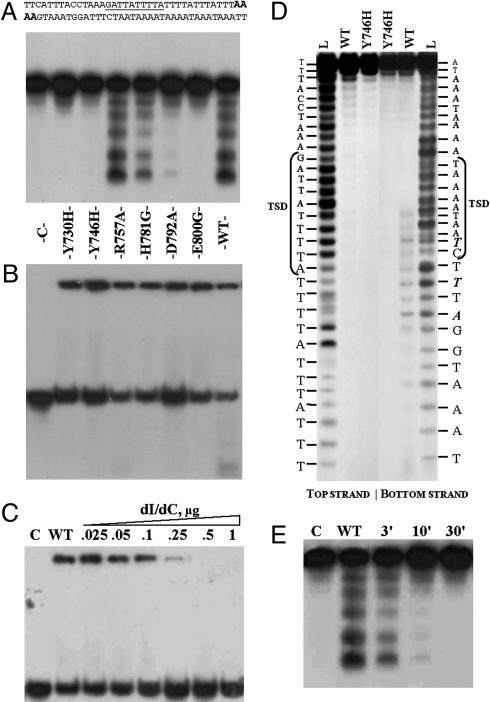
Fig. 3.
Properties of Penelope-encoded EN and its mutant derivatives. (A and B) Cleavage and DNA-binding assays. The 38-bp ps1 target (A Upper) was labeled with [α-32P]dATP at both ends (in bold italics). Lane C, control without protein. The remaining seven lanes in A and B represent cleavage and DNA-binding assays, respectively, for the EN protein and its mutant variants: Y730H (2%); Y746H (0%); R757A (100%); H781G (27%); D792A (5%); E800G (0%); and WT, wild-type Penelope EN (100%); numbers in parentheses designate the relative cleavage activity with respect to WT obtained from PhosphorImager quantitative scans of gel A. To suppress target cleavage, binding reactions in B were performed without Mg2+ and in the presence of EDTA; however, residual cleavage is still visible for the most active R757A and WT proteins. (C) Binding of WT Penelope EN to the ps1 target at different concentrations of poly(dI)·poly(dC). Lane C, control without protein; WT, wild-type Penelope EN in a standard binding reaction with 0.1 mg/ml BSA. In the remaining lanes, increasing amounts of poly(dI)·poly(dC) (from 0.025 to 1 μg) were added to the binding reaction, as indicated. (D) Determination of cleavage sites of Penelope EN. The 34-bp fragment of ps1 was used as a target, with the top and bottom strand labeled as indicated. Lane L, 1-bp DNA ladder; WT, labeled fragments digested by WT EN; Y746H, labeled fragments digested by the EN mutant. Target site duplication (TSD) is shown by brackets, and the most prominent cleavage products, which become visible after a 1-h incubation, are shown in bold italics. (E) Ligation of the labeled ps1 fragment after digestion by the WT Penelope EN. Lane C, labeled fragment without protein; WT, labeled fragment digested with EN. The remaining lanes show ligation of the cleavage products after 3-, 10-, and 30-min incubation with T4 DNA ligase.