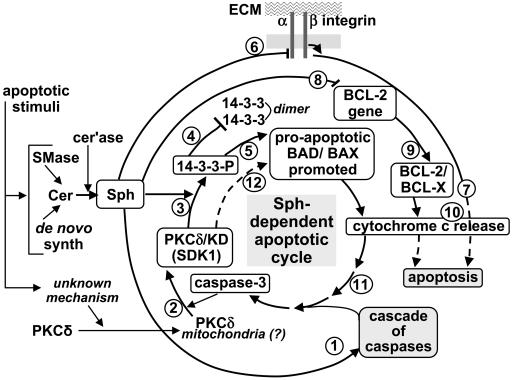
Fig. 4.
Multiple Sph-induced mechanisms leading to apoptosis. Apoptotic stimulation causes (i) a higher Cer level, resulting from higher sphingomyelinase (SMase) activity or its de novo synthesis, followed by release of Sph by ceramidase (cer'ase) and (ii) an unknown mechanism causing translocation of PKCδ and its possible accumulation in mitochondrial membrane. These two events are considered to trigger multiple Sph-induced channels for apoptosis processes 1–12 as described below. (Process 1) Sph activates a “cascade of caspases” leading to caspase 3 activation. (Process 2) Activated caspase-3 cleaves PKCδ to produce PKCδ KD (SDK1). (Process 3) Sph activates SDK1 to phosphorylate 14-3-3 (→ 14-3-3-P). (Processes 4 and 5) 14-3-3 phosphate (14-3-3-P) inhibits 14-3-3 dimer formation (process 4), which in turn inhibits binding of 14-3-3 to proapoptotic BAD/BAX, promoting their proapoptotic effect (process 5). (Processes 6 and 7) Sph inhibits integrin-dependent survival signal, e.g., Akt (process 6), based on interaction of integrin α3β1 with extracellular matrix (ECM) components (e.g., LN10/11). Inhibition of survival signal leads to apoptosis (process 7). (Processes 8 and 9) Sph inhibits BCL-2 gene expression (process 8), thereby inhibiting antiapoptotic BCL-2/BCL-X (process 9). (Processes 10 and 11) Processes 5, 7, and 9 cause release of cytochrome c (process 10), which contributes to caspase-3 activation (process 11). (Process 12) Activated caspase 3 releases PKCδ KD (SDK1), promoting a “vicious cycle” of Sph-induced apoptosis. The overall process is based on enhanced Sph level and translocation of PKCδ. The higher the Sph level, the greater the effect of the cycle through released caspase 3 and released PKCδ KD. Susceptibility of adherent tumor cells to Sph-induced apoptosis is less than that of nonadherent cells, because adherent cells require inhibition of integrin-dependent survival signal by Sph (process 6). Which Sph-induced mechanism is dominant may vary depending on type of cell.