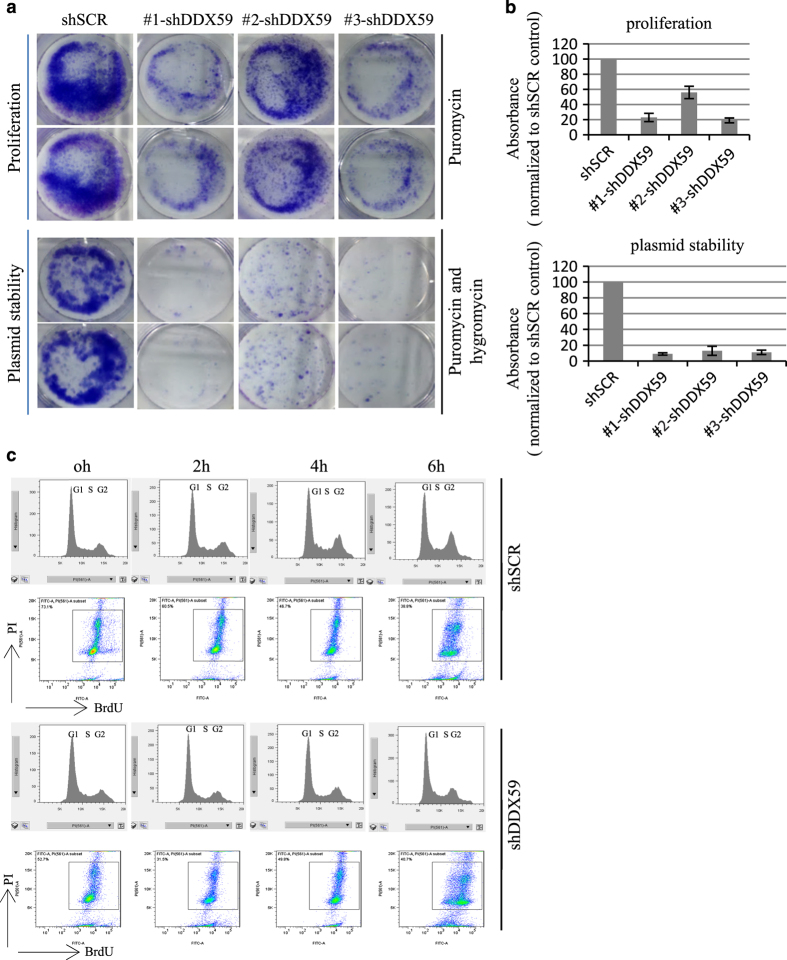
Figure 7.
DDX59 promotes plasmid stability and cell cycle progression. (a) H1299 cells were transduced with lentivirus encoding either shSCR or shDDX59. Two days post infection, cells were transfected with equal amount of p220.2 plasmids, cells were then plated onto six-well dishes in equal cell numbers. For proliferation assay, cells were selected by 5 μg/ml puromycin for 2 days and then changed into 2 μg/ml puromycin and incubated for 1–2 weeks. For plasmid stability test, 10 times more cells were selected by 400 μg/ml of hygromycin B and 5 μg/ml of puromycin for 2 days, then changed into 50 μg/ml of hygromycin B and 2 μg/ml of puromycin and further incubated for 1–2 weeks before performing Giemsa staining. (b) Upper panel: quantitation of foci for the proliferation analysis samples. Bars stand for S.D. from three separate analyses. Lower panel: quantitation of foci for the plasmid stability analysis samples. Bars stand for S.D. from three separate analyses. (c) H1299 cells were transduced with lentivirus encoding either shSCR or shDDX59. Four days post infection, cells were pulsed with BrdU for 6 h, and then chased for 0, 2, 4, and 6 h, respectively. Cells were then analyzed by flow cytometry after incubation with anti-BrdU and PI staining. Cell cycle distribution was obtained for BrdU-positive cells only after gating.