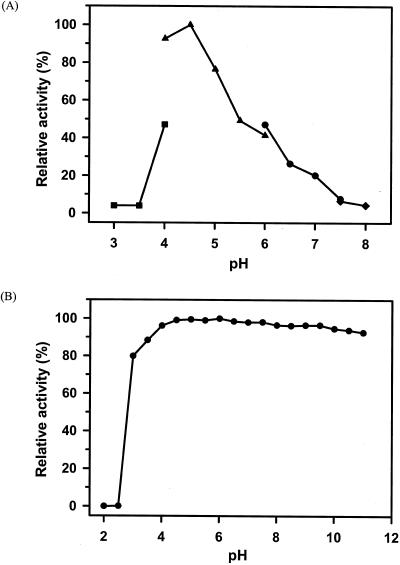
FIG. 3.
Effect of pH on the activity and stability of PFTA. (A) For the determination of optimal pH, the following buffers were used: for pH 3.0 to 4.0, 50 mM sodium citrate (▪); for pH 4.0 to 6.0, 50 mM sodium acetate (▴); for pH 6.0 to 7.5, 50 mM sodium phosphate (•); and for pH 7.5 to 8.0, 50 mM Tris-HCl (♦). The values are shown as percentages of the maximum specific activity of PFTA observed at pH 4.5, which was taken as 100%. (B) To assess the pH stability of PFTA, the enzyme was incubated at the indicated pH in 0.1 M Britton-Robinson buffer and at 37°C for 24 h. The residual activity was measured at 90°C under the standard conditions of the assay. The values are shown as percentages of the original activity, which was taken as 100%.