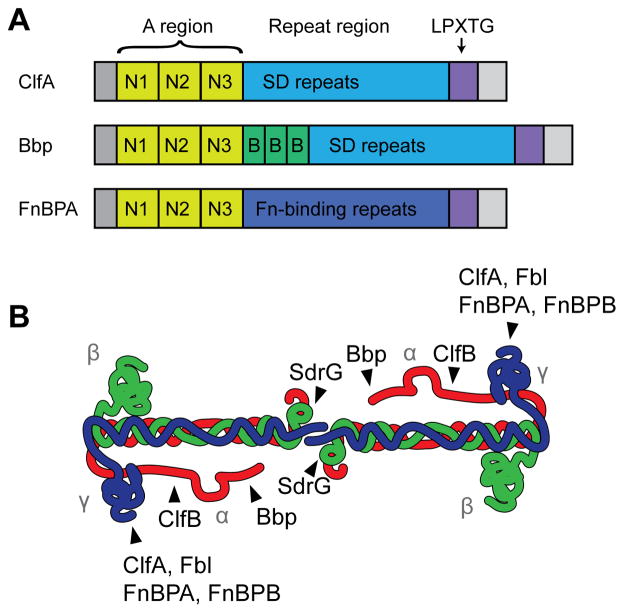
Fig. 3.
MSCRAMM domain organization and interactions with fibrinogen. (A) Representatives of the three known types of fibrinogen-binding MSCRAMMs. The A region, consisting of the N1, N2, and N3 subdomains, is conserved and responsible for fibrinogen binding. The repeat region is variable, with ClfA and Bbp having a series of serine-aspartate dipeptide repeats, while FnBPA contains tandem repeats of a fibronectin-binding domain. Bbp also has three B repeats between the A region and SD repeat region. All of them have a secretion signal at their N-termini and a cell wall anchoring LPXTG sortase signal at their C-termini. S. aureus ClfB and S. lugdunensis Fbl are similar to ClfA, S. aureus SdrE and S. epidermidis SdrG are similar to Bbp (although the number of B repeats is variable), and FnBPA and FnBPB share similar domain architectures. (B) Schematic of fibrinogen, showing where each MSCRAMM binds. Figure is adapted from (Ko and Flick, 2016).