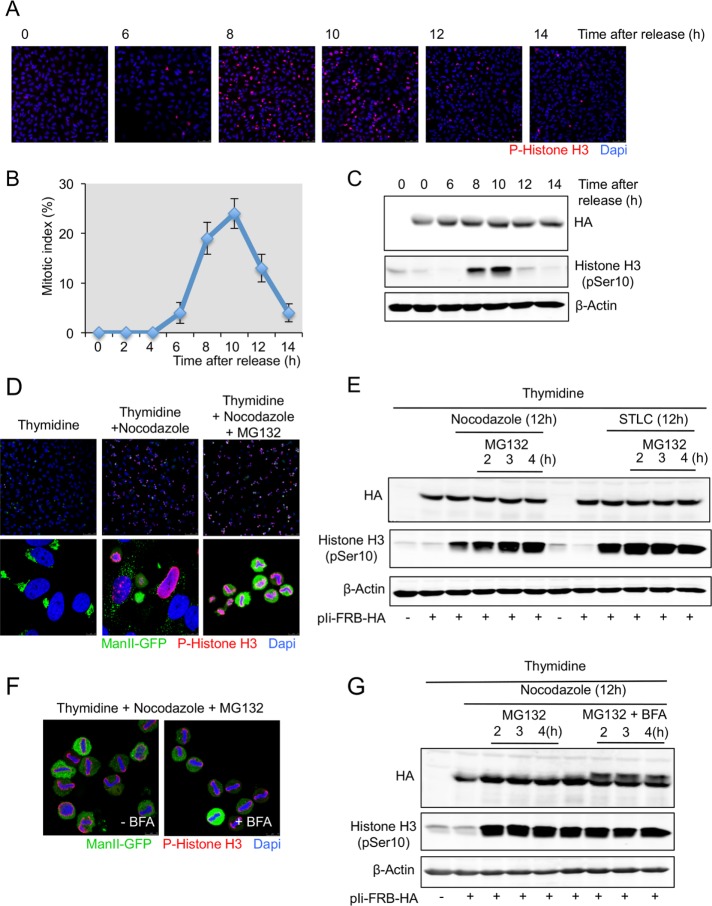
FIGURE 5:
The Ii protein is not O-glycosylated in cells synchronized in prometaphase and metaphase. (A) HeLa cells grown on coverslips were transfected with Ii-FRB-HA plasmid and synchronized in S phase after a double thymidine block. Cells were washed to remove thymidine, and at the indicated times, cells were fixed and stained with an anti–phospho-histone H3 antibody and DAPI and visualized by fluorescence microscopy (scale bar, 100 μm). (B) The percentage of cells in mitosis (mitotic index) was determined by staining DNA with DAPI and an anti–phospho-histone H3 antibody at the indicated times after a double thymidine block and release. We counted 400 cells for each time point (mean ± SD, n = 3). (C) HeLa cells were transfected or not with Ii-FRB-HA plasmid and synchronized in S phase after a double thymidine block. Cells were washed to remove thymidine and lysed at the indicated times cells. Total cell lysates were analyzed by Western blotting with an anti-HA antibody and an anti–phospho-histone H3 antibody. Western blotting with an anti–β-actin antibody was used as a loading control. (D) HeLa cells expressing ManII-GFP grown on coverslip were transfected with Ii-FRB-HA plasmid and synchronized in S phase (double thymidine block), prometaphase (double thymidine block plus nocodazole), and metaphase (double thymidine block plus nocodazole plus MG132 during 2 h). Then cells were fixed and analyzed by immunofluorescence with an anti–phospho-histone H3 antibody and DAPI. Top, low magnification (scale bar, 100 μm). Bottom, high magnification (scale bar, 10 μm). (E) HeLa cells expressing ManII-GFP were transfected with Ii-FRB-HA plasmid and synchronized in S phase (double thymidine block), prometaphase (double thymidine block plus nocodazole or STLC), and metaphase (double thymidine block plus nocodazole or STLC plus MG132). At the indicated time, cells were lysed and total cell lysates analyzed by Western blotting with an anti-HA antibody and an anti–phospho-histone H3 antibody. Western blotting with an anti–β-actin antibody was used as a loading control. (F) HeLa cells expressing ManII-GFP grown on coverslips were transfected with Ii-FRB-HA plasmid and synchronized in metaphase (double thymidine block plus nocodazole plus MG132 during 2 h). BFA was added to the medium at the same time as MG132. Then cells were fixed and analyzed by immunofluorescence with an anti–phospho-histone H3 antibody and DAPI (scale bar, 10 μm). (G) HeLa cells expressing ManII-GFP were transfected with Ii-FRB-HA plasmid and synchronized in S phase (double thymidine block), prometaphase (double thymidine block plus nocodazole), and metaphase (double thymidine block plus nocodazole plus MG132 with or without BFA). At the indicated time, cells were lysed and total cell lysates analyzed by Western blotting with an anti-Ha antibody and an anti–phospho-histone H3 antibody. Western blotting with an anti–β-actin antibody was used as a loading control.