FIGURE 1:
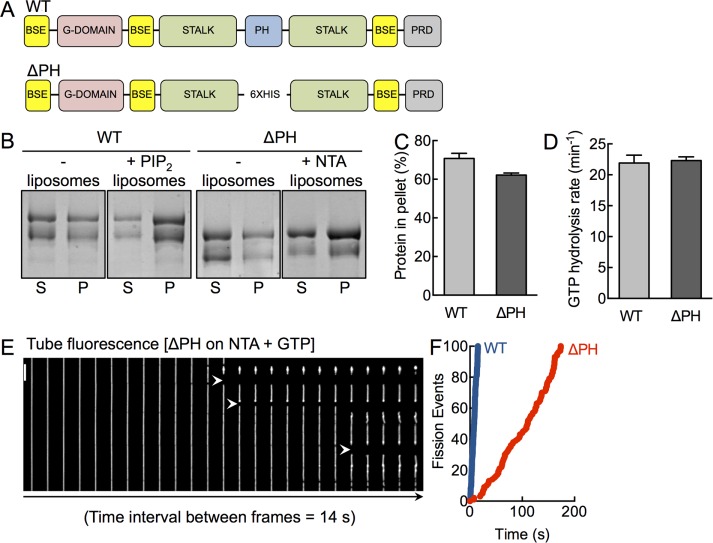
A functionally active dynamin construct lacking the PHD. (A) Schematic representation of dynamin showing the bundle-signaling element (BSE), GTPase domain (G-domain), stalk, pleckstrin-homology domain (PHD), and proline-arginine rich domain (PRD). The ΔPH construct has the entire PH domain deleted and replaced with a 6xHis linker. (B) Coomassie brilliant blue–stained SDS–PAGE of WT and ΔPH in the absence and presence of PIP2- and NTA-containing liposomes after sedimentation, showing protein amounts in the supernatant (S) and pellet (P) fractions. The low–molecular weight band in both constructs reflects a proteolyzed form that partially lacks the C-terminus, which we confirmed using Western blotting with streptavidin-HRP, which binds the engineered StrepII tag at the C-terminus (Supplemental Figure S1). (C) Densitometric analysis of gels (both bands) shown in B, indicating the fraction of protein in the pellet. Data represent mean ± SD (N = 2). (D) Assembly-stimulated GTP hydrolysis rates of WT and ΔPH in presence of PIP2- or NTA-containing liposomes, respectively. Data represent mean ± SD (N = 3). (E) Time-lapse sequence showing addition of ΔPH to SMrT templates in the presence of GTP. White arrowheads mark sites of scission. Scale bar, 5 μm. (F) Cumulative fission rates of WT (blue) and ΔPH (red). Data represent pooled analysis (n ≥ 19, N = 3).