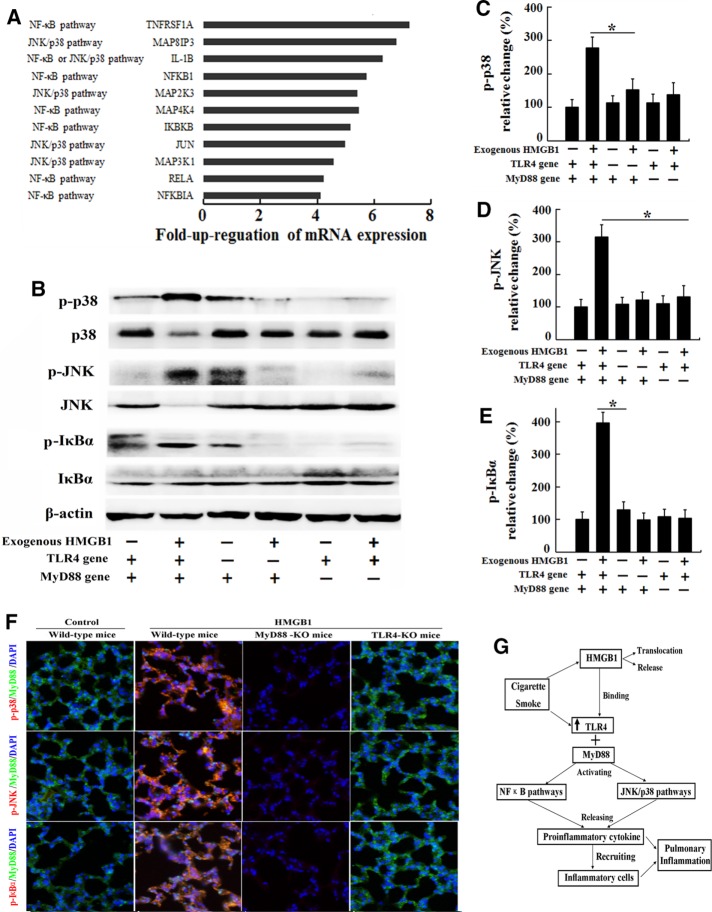
FIGURE 6:
Signal transduction is activated in lungs exposed to the inflammatory CS milieu. C57BL/6 mice received an intravenous injection of 200 μg/kg rHMGB1 or vehicle (PBS) via the tail vein each day (n = 5 for each group) during acute (3 d) exposure to CS. Three days later, the lungs were collected for the real-time PCR assay. (A) Genes involved in TLR4 signaling and molecules associated with specific downstream pathways that exhibited an at least fourfold increase in expression in the lungs from the rHMGB1-treated group compared with the vehicle group. MTE cells harvested from wild-type mice, TLR4-KO mice, or MyD88-KO mice were stimulated with human rHMGB1 (100 ng/ml) or vehicle (PBS) for 24 h. After stimulation, the cells were collected. (B) The levels of phosphorylated MAPKs p38, JNK, and IκBα were evaluated by Western blotting. The gels were run under the same experimental conditions. The expression levels of (C) phospho-p38, (D) phospho-JNK (D), and (E) phospho-IκBα were normalized to the levels of total p38, total JNK, and total IκBα, respectively. Wild-type mice, TLR4-KO mice, and MyD88-KO mice received an intravenous injection of 200 μg/kg rHMGB1 or control (PBS) via the tail vein each day during acute (3 d) exposure to CS (n = 5 for each group). (F) Immunohistochemical staining for phospho-p38, phospho-JNK, and phospho-IκBα in the lung tissue. (G) Diagram summarizing the mechanism by which HMGB1 mediates CS-induced pulmonary inflammation in the mouse. The results are displayed as means ± SD. from three independent experiments. *p < 0.05.