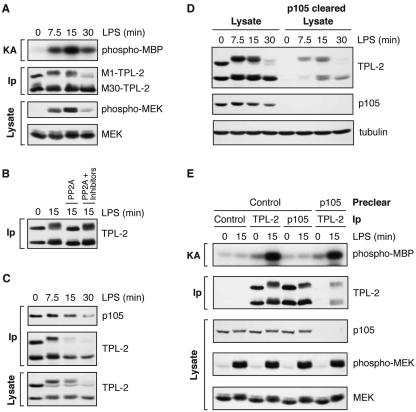
FIG. 1.
LPS stimulation induces a p105-free pool of TPL-2 which activates MEK. BMDMs (BALB/c) were stimulated with LPS for the indicated times. (A) TPL-2 was immunoprecipitated from cell lysates with anti-TPL-2 antibody, and its MEK kinase activity was determined by coupled MEK/ERK kinase assay. Labeled MBP substrate was visualized by autoradiography after SDS-PAGE, and the levels of immunoprecipitated TPL-2 were determined by Western blotting. Lysates were also subjected to Western blotting with an anti-phospho-(S217/S221)-MEK-1/2 antibody (phospho-MEK) to determine activation of endogenous MEK by LPS. (B) TPL-2 was immunoprecipitated from cell lysates. Beads were incubated with 0.5 U of PP2A, 0.5 U of PP2A plus the phosphatase inhibitors NaF and okadaic acid (PP2A + Inhibitors), or control buffer. Immunoprecipitated TPL-2 was revealed by Western blotting. (C) Cell lysates and anti-p105 immunoprecipitates were subjected to Western blotting. (D) Total cell lysates (lysates) and p105 cleared lysates were subjected to Western blotting for TPL-2 and p105. Equal protein loading was confirmed by probing for α-tubulin. (E) Cell lysates were immunoprecipitated with anti-TPL-2 antibody, anti-p105N antibody, or control Ig (control) after first immunodepleting with either anti-p105N antibody (p105) or control Ig (control) as indicated. Immunoprecipitates were assayed for associated MEK kinase activity and TPL-2 levels as for panel A. Cell lysates were subjected to Western blotting to confirm depletion of p105 and activation of MEK phosphorylation by LPS.