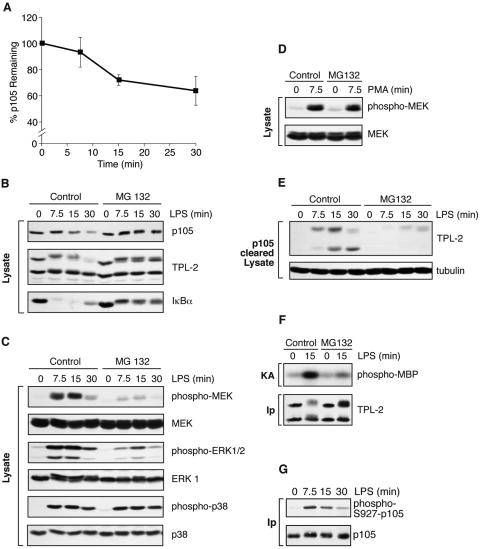
FIG. 2.
Proteasome activity is required for LPS activation of TPL-2. (A) BMDMs were simulated with LPS for the indicated times. Total p105 protein levels in cell lysates were determined by Western blotting and quantified using a Fuji Image reader. Data are presented as means (± SEM; n = 3), normalized against α-tubulin. (B to E) BMDMs were preincubated with the proteasome inhibitor MG132 (40 μM) or DMSO vehicle control for 30 min and then stimulated with LPS or PMA for the indicated times. Total cell lysates were subjected to Western blotting for the indicated proteins. LPS activation of endogenous MEK, ERK, and p38 was monitored with the appropriate phospho-specific antibodies. (E) Cell lysates were immunodepleted of p105 prior to Western blotting for TPL-2 and α-tubulin. (F) BMDMs were treated with MG132 (40 μM) prior to stimulation with LPS for 15 min. TPL-2 was immunoprecipitated from total cell lysates and then assayed for MEK kinase activity as for Fig. 1A. (G) BMDMs, pretreated with 40 μM MG132, were simulated with LPS for the indicated times. p105 was immunoprecipitated from total cell lysates, subjected to Western blotting, and probed with a specific anti-phospho-peptide antibody to monitor p105 serine 927 phosphorylation.