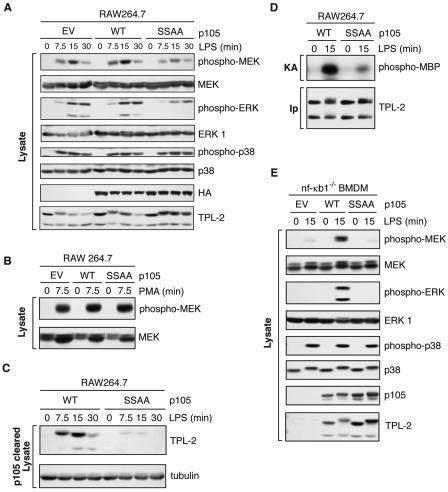
FIG. 4.
LPS activation of TPL-2 is dependent on signal-induced p105 proteolysis. (A to D) RAW 264.7 cells stably transfected with vectors encoding wild-type HA-p105 (WT), HA-p105S927A,S932A (SSAA), or with no insert (EV) were stimulated with LPS or PMA for the indicated times. (A and B) Total cell lysates were subjected to Western blotting, and phospho-specific antibodies were used to monitor MEK, ERK, and p38 activation. (C) Lysates were immunodepleted of p105 and subjected to Western blotting for TPL-2 and α-tubulin. (D) TPL-2 was immunoprecipitated from lysates of RAW264.7 cells expressing HA-p105 (WT) or HA-p105S927A,S932A (SSAA), and MEK kinase activity was determined by a coupled MEK/ERK kinase assay as for Fig. 1A. (E) BMDMs generated from NF-κB1−/− mice were infected with recombinant retroviruses encoding wild-type FL-p105 (WT), FL-p105S927A,S932A (SSAA), or with no insert (EV). Cells were stimulated with LPS (10 ng/ml) for 15 min or left untreated, and total cell lysates were subjected to Western blotting for the indicated proteins. Endogenous MEK, ERK, and p38 activation was assayed using phospho-specific antibodies.