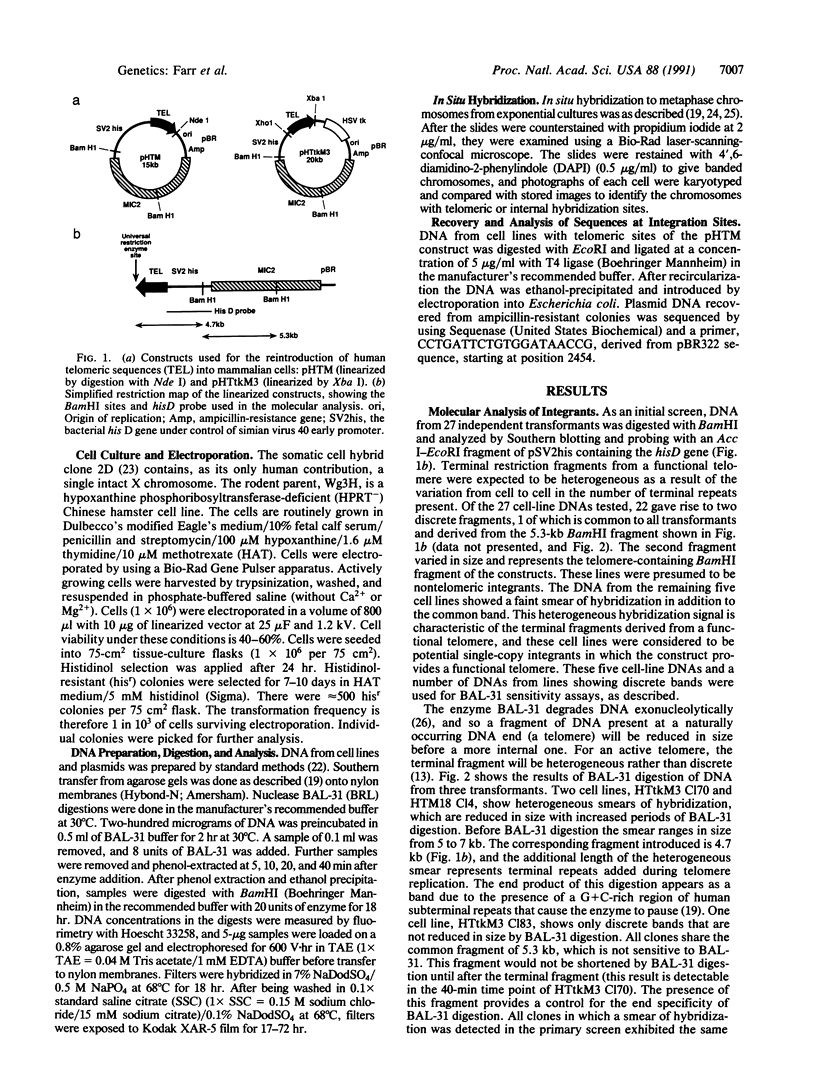
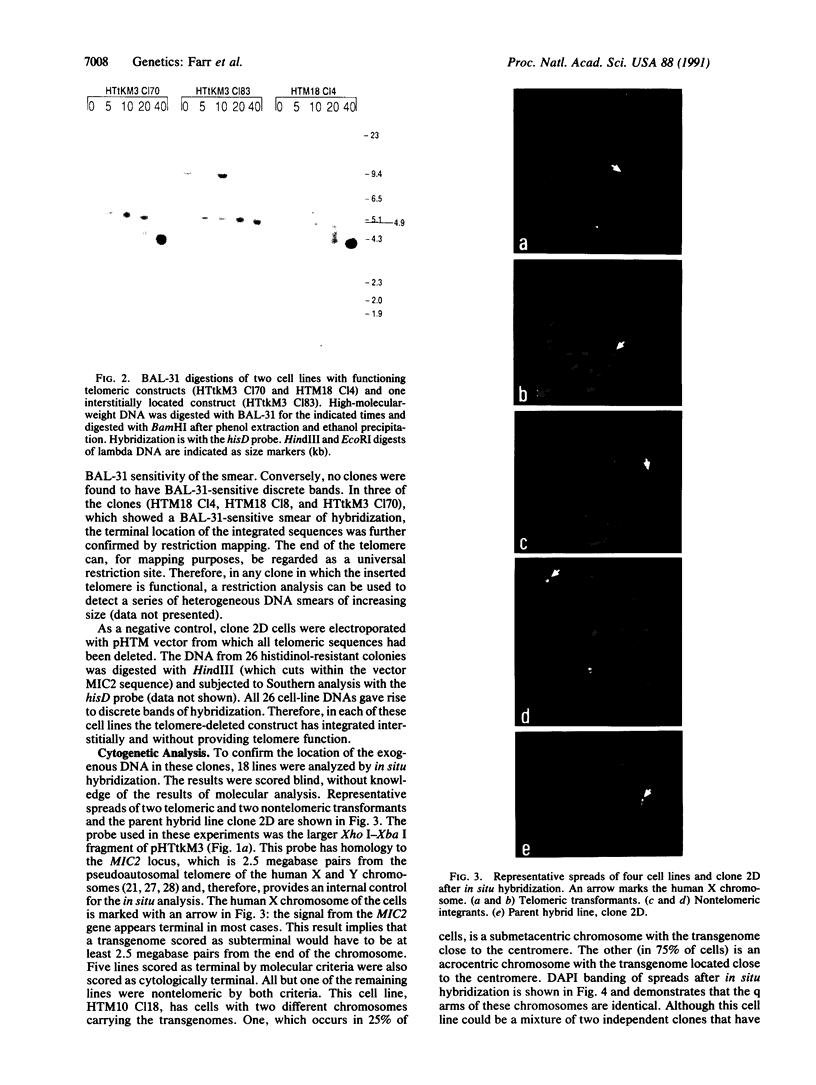
Abstract
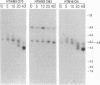
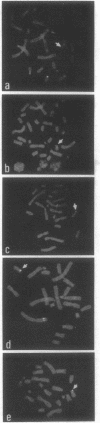
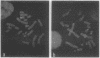
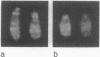
Telomeric sequences of eukaryotes consist of short tandem repeats organized in arrays of variable length in which the guanine-rich strand runs 5'----3' toward the chromosomal end. The terminal repeats in yeast are the only elements necessary for telomere function in this organism. To test whether mammalian terminal repeats can function after reintroduction into a mammalian cell, a repeat-containing terminal fragment from a human chromosome was electroporated into a hamster-human hybrid cell line. In 6 of 27 independent transformants analyzed, the introduced sequences were found at the ends of chromosomes, based on all available criteria. Terminal restriction-fragment heterogeneity and the survival of these chromosomes demonstrate that these telomeres are functional. Cytogenetic evidence from one of these cell lines suggests that chromosome breakage with healing at the integration site is the mechanism responsible for the terminal location.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Gosden J. R., Cross S. H., Cranston G., Rout D., Sugawara N., Szostak J. W., Fantes P. A., Hastie N. D. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988 Apr 14;332(6165):656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Boggs S. S., Gregg R. G., Borenstein N., Smithies O. Efficient transformation and frequent single-site, single-copy insertion of DNA can be obtained in mouse erythroleukemia cells transformed by electroporation. Exp Hematol. 1986 Nov;14(10):988–994. [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Brown W. R. A physical map of the human pseudoautosomal region. EMBO J. 1988 Aug;7(8):2377–2385. doi: 10.1002/j.1460-2075.1988.tb03082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R. Molecular cloning of human telomeres in yeast. Nature. 1989 Apr 27;338(6218):774–776. doi: 10.1038/338774a0. [DOI] [PubMed] [Google Scholar]
- Cheng J. F., Smith C. L., Cantor C. R. Isolation and characterization of a human telomere. Nucleic Acids Res. 1989 Aug 11;17(15):6109–6127. doi: 10.1093/nar/17.15.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980 Oct 9;287(5782):504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Smith B. A. Variability at the telomeres of the human X/Y pseudoautosomal region. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):213–219. doi: 10.1101/sqb.1986.051.01.026. [DOI] [PubMed] [Google Scholar]
- Cross S. H., Allshire R. C., McKay S. J., McGill N. I., Cooke H. J. Cloning of human telomeres by complementation in yeast. Nature. 1989 Apr 27;338(6218):771–774. doi: 10.1038/338771a0. [DOI] [PubMed] [Google Scholar]
- Cross S., Lindsey J., Fantes J., McKay S., McGill N., Cooke H. The structure of a subterminal repeated sequence present on many human chromosomes. Nucleic Acids Res. 1990 Nov 25;18(22):6649–6657. doi: 10.1093/nar/18.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. J., Darling S. M., Thomas N. S., Goodfellow P. N. A pseudoautosomal gene in man. Science. 1986 Nov 7;234(4777):740–743. doi: 10.1126/science.2877492. [DOI] [PubMed] [Google Scholar]
- Goss S. J., Harris H. Gene transfer by means of cell fusion I. Statistical mapping of the human X-chromosome by analysis of radiation-induced gene segregation. J Cell Sci. 1977 Jun;25:17–37. doi: 10.1242/jcs.25.1.17. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990 Nov 16;63(4):751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Cech T. R. Chromatin structure of the molecular ends of Oxytricha macronuclear DNA: phased nucleosomes and a telomeric complex. Cell. 1984 Sep;38(2):501–510. doi: 10.1016/0092-8674(84)90505-1. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Claus T. E., Szostak J. W. Characterization of two telomeric DNA processing reactions in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4642–4650. doi: 10.1128/mcb.8.11.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Construction and behavior of circularly permuted and telocentric chromosomes in Saccharomyces cerevisiae. Mol Cell Biol. 1986 Sep;6(9):3166–3172. doi: 10.1128/mcb.6.9.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon C. S. Yeast chromosome replication and segregation. Microbiol Rev. 1988 Dec;52(4):568–601. doi: 10.1128/mr.52.4.568-601.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Weissenbach J. Physical mapping of the human pseudo-autosomal region; comparison with genetic linkage map. EMBO J. 1988 Aug;7(8):2369–2376. doi: 10.1002/j.1460-2075.1988.tb03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Dani G. M., Spear B. B., Zakian V. A. Elaboration of telomeres in yeast: recognition and modification of termini from Oxytricha macronuclear DNA. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1475–1479. doi: 10.1073/pnas.81.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Zakian V. A. Recombination occurs during telomere formation in yeast. Nature. 1989 Feb 2;337(6206):429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- Riethman H. C., Moyzis R. K., Meyne J., Burke D. T., Olson M. V. Cloning human telomeric DNA fragments into Saccharomyces cerevisiae using a yeast-artificial-chromosome vector. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6240–6244. doi: 10.1073/pnas.86.16.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W., Connelly C., Hieter P. Physical mapping of large DNA by chromosome fragmentation. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6027–6031. doi: 10.1073/pnas.85.16.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Collins C., Robbins C., Magenis R. E., Delaney A. D., Gray J. W., Hayden M. R. Characterization and organization of DNA sequences adjacent to the human telomere associated repeat (TTAGGG)n. Nucleic Acids Res. 1990 Jun 11;18(11):3353–3361. doi: 10.1093/nar/18.11.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]