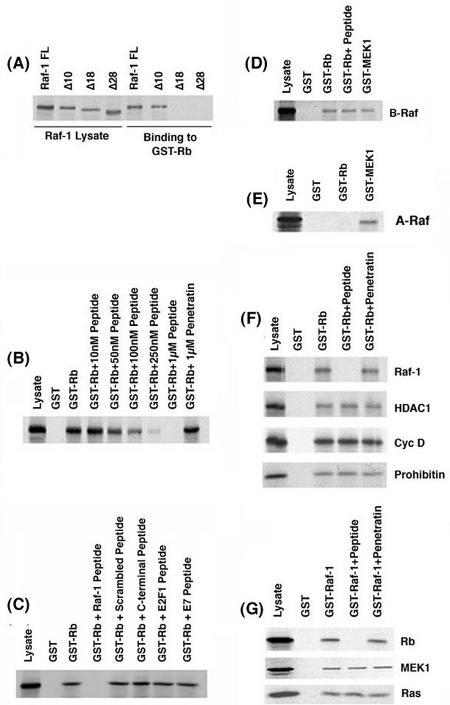
FIG. 1.
Binding of 35S-labeled Raf-1 deletion fragments to GST-Rb in vitro. (A) 35S-Raf-1WT and the deletion fragments 35S-Raf-1Δ10, 35S-Raf-1Δ18, and 35S-Raf-1Δ28 were tested for binding to GST-Rb or unprimed GST beads. Lysate lane has one-fourth of the protein used for binding. (B) Dose-dependent inhibition of the binding of Raf-1 to GST-Rb by the Raf-1 peptide in a GST pull-down assay. A 1 μM concentration of an unrelated peptide (penetratin) was used as a negative control. (C) The Rb-Raf-1 interaction is abolished by 1 μM of the Raf-1 peptide but is unaffected by a 1 μM concentration of a scrambled peptide, a control peptide from the C terminus of Raf-1, or peptides corresponding to the Rb-binding domain on E2F1 (E2F peptide) and HPV E7 protein (E7 peptide). (D) The Raf-1 peptide is unable to disrupt the binding of B-Raf to GST-Rb and GST-MEK1 in vitro. (E) 35S-A-Raf does not bind to GST-Rb but binds to the positive control GST-MEK1. (F) The Raf-1 peptide specifically disrupts the Rb-Raf-1 interaction but does not affect the binding of HDAC1, cyclin D, and prohibitin to GST-Rb. (G) The Raf-1 peptide does not affect the interaction of other Raf-1-binding proteins such as MEK1 and Ras to GST-Raf-1.