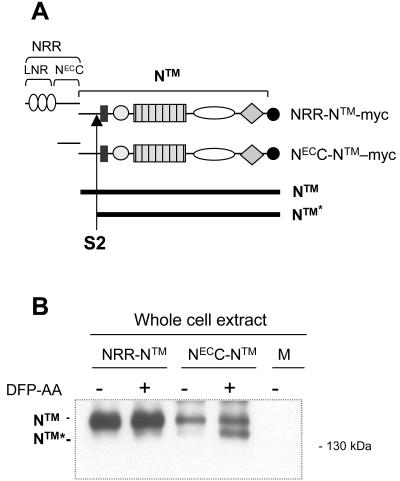
FIG. 6.
The LNR domain of hN1 confers resistance to proteolysis at the S2 site. (A) Schematic representation of the hN1 polypeptides used in the proteolysis experiments, noting the position of the myc epitope tag, the site of S2 cleavage, and the difference in polypeptide length between native NTM and the product after S2 cleavage, denoted NTM*. (B) The S2 proteolysis product NTM* is generated when the LNR domain is deleted from hN1. U2OS cells transfected with the indicated hN1 variants were incubated in the presence of a presenilin inhibitor (DFP-AA). NTM and NTM* polypeptides were then recovered by immunoprecipitation with an anti-myc antibody, resolved by SDS-PAGE under reducing conditions, and detected by Western blotting using anti-myc.