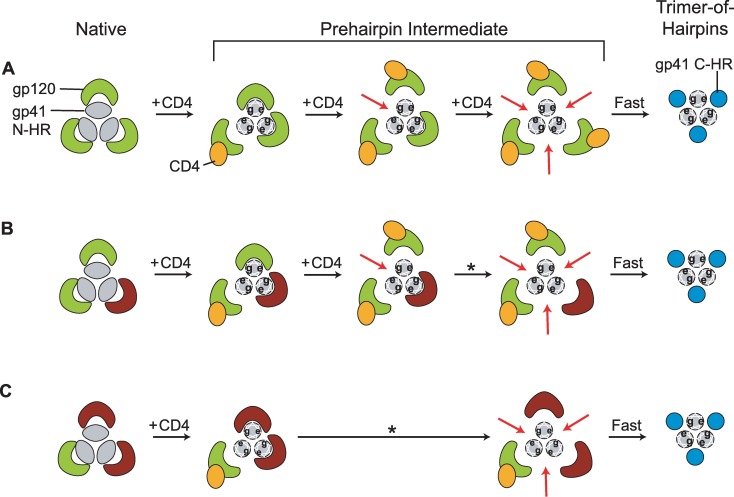
Fig 8. Hypothetical model of the early structural changes in Env leading to exposure of the N-HR coiled coil.
(A) Proposed transitions for an Env trimer that encounters three CD4 molecules. In the native state, gp120 subunits (green) constrain the N-HR segments (grey) in a metastable conformation in which the coiled coil is only partially formed (schematically represented as ovals). The binding of the first CD4 (orange) alters trimer structure enabling formation of the full N-HR coiled coil (schematically represented as circles with e- and g-positions of the heptad repeat designated). The CD4-bound gp120 subunit disengages but only partially exposes C-peptide binding sites on either side of its cognate N-HR helix. The remaining two gp120 subunits remain closely associated with the coiled coil, thereby blocking C-peptide access to all three binding sites. The second CD4-gp120 interaction causes this gp120 subunit to disengage, opening up a single C-peptide binding site on the N-HR coiled coil (designated by the red arrow). The other two C-peptide binding sites remain partially occluded by the one gp120 that remains attached. Upon association of the third CD4, the last gp120 disengages and the N-HR coiled coil with its three C-peptide binding sites becomes fully exposed. Ultimately, these sites become occupied by gp41 C-HR regions (blue) as Env transitions into its fusogenic trimer-of-hairpins conformation. The model describes the putative transitions of homotrimeric Envs, including the Q552R/N637K variant of Fig 7. (B and C) Proposed transitions for an Env trimer that encounters only two (B) or one (C) CD4 molecule. The gp120 subunits that do not interact with CD4 are colored brown. The asterisk indicates a spontaneous, CD4-independent conformational change. The models describe the putative transitions of A2B (B) and AB2 (C) heterotrimers.