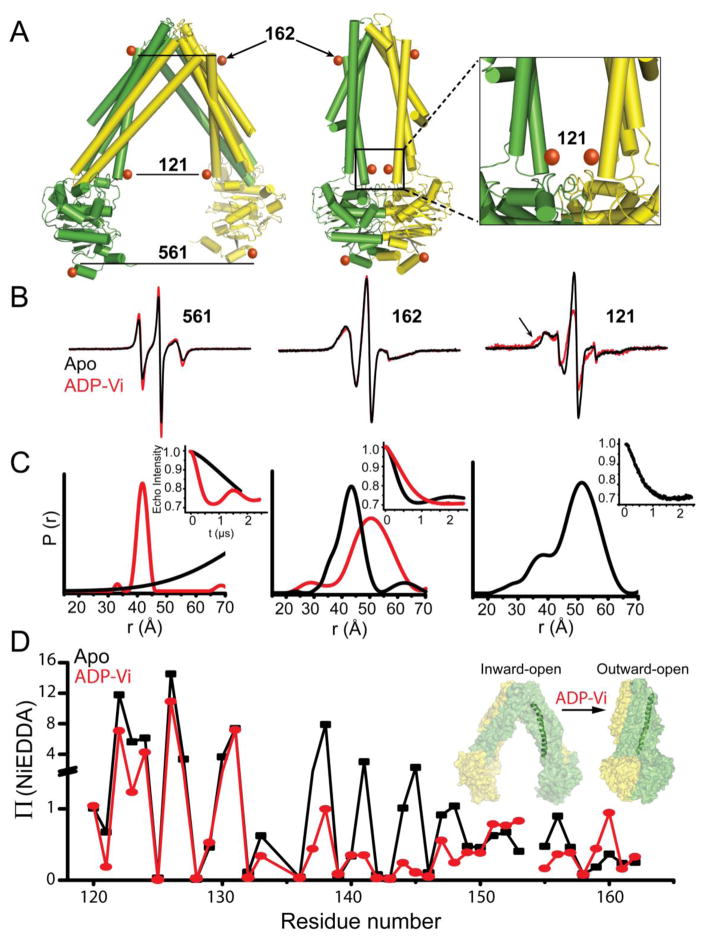
Figure 7. Correlation of global structural rearrangements with local helix packing in MsbA.
(A) Model of the MsbA homodimer in the open, Apo (PDB 3B5W) and the closed, AMP-PNP-bound (PDB 3B60) states showing symmetry-related sites for spin label incorporation. Individual monomers are identified by the color scheme. (B) EPR spectra of spin labels at these positions and the corresponding distance distributions (C) in the Apo and ADP-Vi-bound states (trapped post-hydrolysis). Labels at 561 and 162 show opposite distance changes between states, consistent with rigid body movement of helices in an alternating access mechanism. Although separated by ~50Å in the Apo state (C), spin labels at site 121 are within 20Å in the ADP-Vi-bound state as indicated by broadening of the EPR lineshape (arrow in B). (D) Formation of a closed conformation on the intracellular side according to distance analysis is consistent with changes in the NiEDDA accessibility profile of transmembrane helix 3 induced by ADP-Vi.