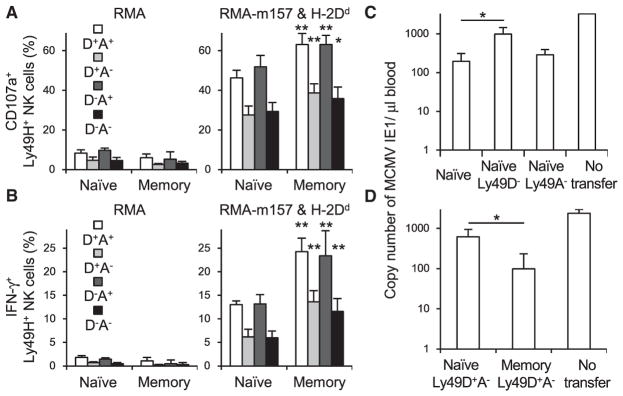
Figure 6. B10.D2 Memory Ly49H+ NK Cells Exert Augmented Effector Functions and Provide Protective Host Defense against Re-challenge with MCMV.
B10.D2 Ly49H+ NK cells were transferred into Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and then infected with 1 × 104 pfu MCMV. Memory Ly49H+ NK cells were enriched from spleens of recipient mice 25–29 days pi.
(A and B) Degranulation (A) and IFN-γ production (B) of B10.D2 naive and memory Ly49H+ NK cell subsets expressing Ly49D and or Ly49A after co-culture with RMA or RMA cells expressing m157 and H-2Dd. Data are pooled from three experiments (n = 9–11 in each group). *p < 0.05, **p < 0.005 versus naive NK cell subsets.
(C) 50,000 B10.D2 naive Ly49H+ NK cells, Ly49D−Ly49H+ NK cells, and Ly49A−Ly49H+ NK cells were purified from spleens of uninfected mice and transferred into Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and then infected with MCMV. The copy number of MCMV IE1 gene in the blood on day 7 pi was analyzed by qPCR.
(D) B10.D2 naive Ly49D+Ly49A−Ly49H+ NK cells were purified from spleens of uninfected mice. B10.D2 memory Ly49D+Ly49A−Ly49H+ NK cells were purified from spleens of recipient mice 25 days pi. 20,000 B10.D2 naive and memory Ly49D+Ly49A−Ly49H+ NK cells were transferred into Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and then infected with MCMV. The copy number of MCMV IE1 gene in the blood on day 7 pi was analyzed by qPCR. Data are pooled from two experiments (n = 5–8 in each group).
*p < 0.05. Error bars show SEM.