Figure 1.
Cep104 Contains a Canonical Tubulin-Binding TOG Domain
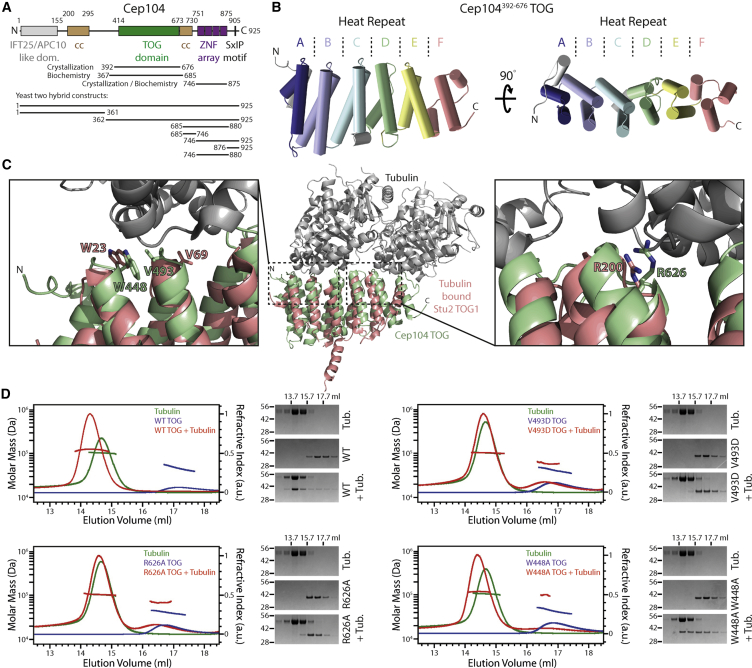
(A) Domain overview of human Cep104. Lines indicate constructs that were used in this work.
(B) Ribbon representation of the Cep104 TOG structure. Helices are displayed as cylinders. The six HEAT repeats that constitute the TOG domain are colored individually. Note the partial distortion of HEAT repeat D and F and the slight curvature of the TOG domain.
(C) Middle: ribbon representation of TOG1 of yeast Stu2 (red) bound to a tubulin dimer (gray), PDB: 4ffb. Stu2 TOG1 is overlaid with our Cep104 TOG structure (green), showing the overall similarity of the TOG domain fold. Left and right: detailed views of the Stu2 TOG1-tubulin-binding interfaces with those residues labeled that were previously found to be critical for tubulin binding by Stu2. Note that these residues are conserved in Cep104 TOG and found in similar positions.
(D) Cep104 TOG binds tubulin in solution. SEC-MALS chromatograms of human Cep104 TOG constructs, run alone or in the presence of tubulin. The horizontal lines indicate the molar masses derived from the refractive index and light-scattering signals as described in Experimental Procedures. On the right side of the chromatograms, Coomassie-stained SDS-PAGE gels show the corresponding peak fractions. The approximate elution volumes are indicated above the gels. The tubulin-alone run is identical in all chromatograms and gels and is only shown repetitively to allow an easier comparison.
See also Figures S1 and S2.