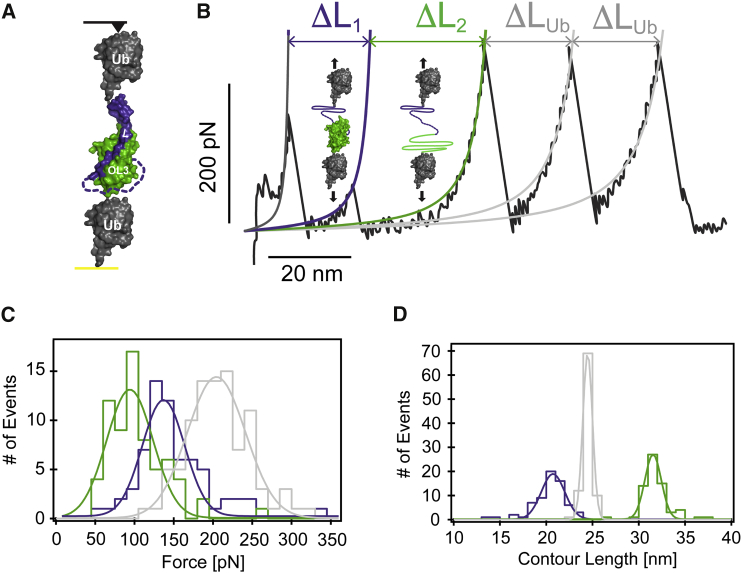
Figure 6.
Fold Complementation by the Myomesin L Linker Protects the Mechanically Labile OL3 Domain
(A) Schematics of the ubiquitin-L-(connector)-OL3-ubiquitin polyprotein used in the single-molecule mechanical experiment. The 43 amino acid flexible connector covalently joining L to OL3 is depicted as a dashed line. The color code for L and OL3 is as in previous images. Ubiquitin (Ub) molecules bracketing the complex are in gray while the gold substrate and the atomic force microscopy (AFM) cantilever tip are shown in yellow and black, respectively.
(B) Typical force-extension trace for the ubiquitin-L-(connector)43-OL3-ubiquitin polyprotein exhibiting a saw-tooth pattern of unfolding events that does not follow a hierarchy in the mechanical stability: the first unfolding event occurs at higher forces than the second unfolding event. The unfolding of the ubiquitin modules, occurring at forces of ∼200 pN and characterized by an increment of contour length of ΔLUb ∼24.5 nm, ensures the single-molecule nature of the experiment.
(C) Histogram of unfolding forces. The first two events can be readily identified in light of their different mechanical stability and increment in contour length. While the first peak occurs at forces as high as 129.4 ± 27.0 pN (n = 66), the second peak unfolds at the markedly lower force of 86.6 ± 29.1 pN (n = 68).
(D) Histogram of contour length increase: ΔL1 = 20.2 ± 1.2 nm (n = 66) and ΔL2 = 31.0 ± 0.9 nm (n = 68). In (C) and (D), colored curves are Gaussian fits.