Figure 5.
FusA Structural Determinants for Carbohydrate Binding Are Involved in FOS Import
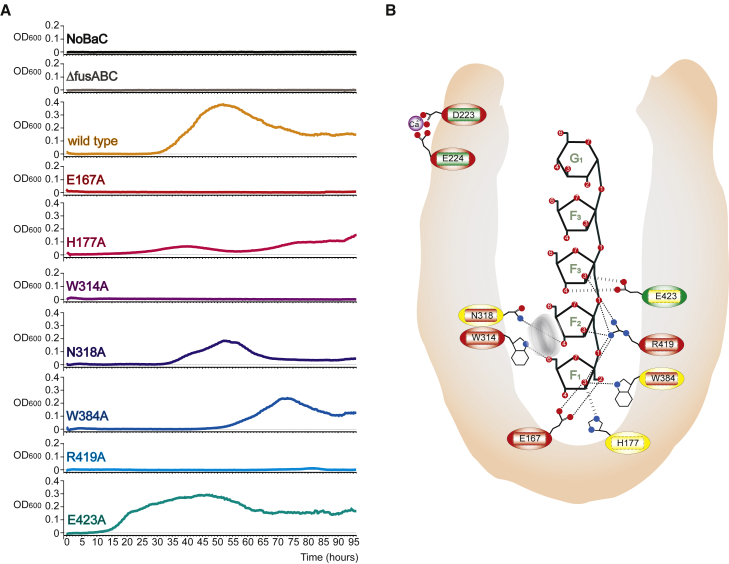
(A) Growth curves of S. pneumoniae on defined medium containing nystose as a sole carbon source. Endogenous FusA was mutated as described for each panel. An optical density at 600 nm (OD600) for wild-type and every mutant was measured every 20 min for 96 hr, showing that most of the mutants have impaired growth on nystose.
(B) Schematic representation of the FusA-FOS-binding site, highlighting the residues involved in FOS binding and import: dotted lines indicate hydrogen bonds or polar interactions, while the gray ring represents the stacking interaction of Trp314 on F2. Mutation of these amino acids affect strongly (red), weakly (yellow), or do not affect (green) binding (inner rectangle) or import (outer circle).