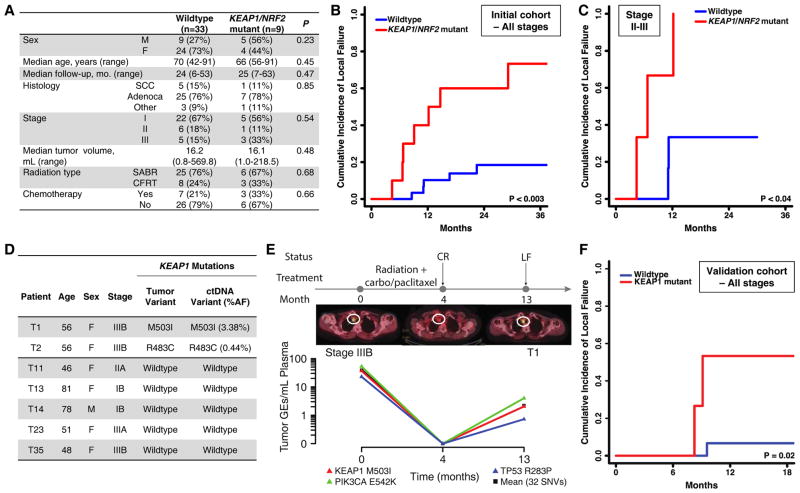
Figure 7. KEAP1/NRF2 mutation status predicts local failure after radiotherapy in human NSCLC.
(A) Clinical characteristics of a cohort of stage I–III NSCLC patients treated with curative intent RT and analyzed for KEAP1/NRF2 mutation status using targeted next generation sequencing.
(B–C) Association of KEAP1/NRF2 mutations with local failure in NSCLC patients treated with radiotherapy. (B) Entire cohort. (C) Stages II–III patients treated with conventionally fractionated radiotherapy or chemoradiotherapy.
(D) Non-invasive identification of KEAP1 mutation status in circulating tumor DNA (ctDNA) isolated from pre-treatment plasma samples of 7 patients from the cohort in (A) using CAPP-Seq.
(E) Monitoring of ctDNA in a KEAP1 mutant patient from (D) treated with chemoradiation. CR, complete response; LF, local failure; carbo, carboplatin.
(F) Clinical characteristics of a validation cohort of stage I–III NSCLC patients treated with curative intent RT and analyzed for KEAP1/NRF2 mutation status using targeted next generation sequencing.