Fig. 1.
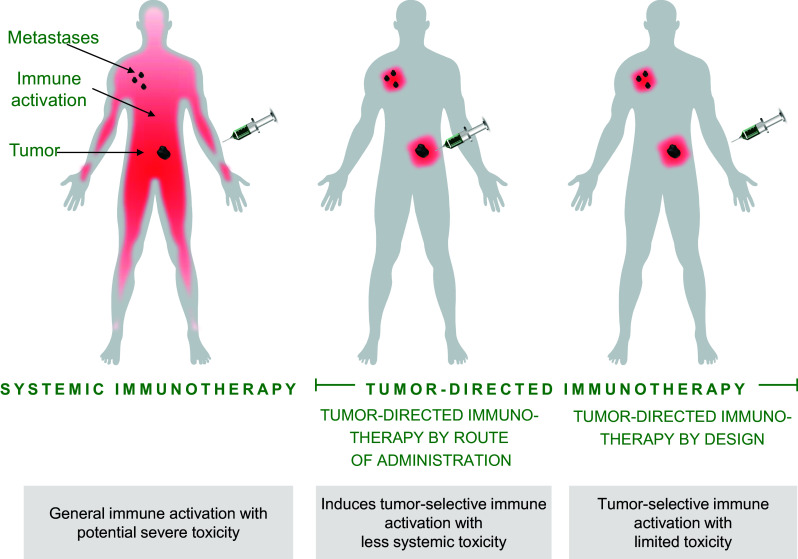
Illustration of tumor-directed immunotherapy (also termed in situ vaccination) compared to systemic immunotherapy. Intravenous administration of agonistic or checkpoint blocking antibodies activates tumor-directed T cells generating an anti-tumor response. However, these treatments can also induce cytokine release, cause liver problems, and activate autoreactive T cells, resulting in immune-related adverse events. Tumor-directed immunotherapy aims to direct immune activation to the tumor and tumor-draining lymph node axis. Activated tumor-directed T cells have the potential to migrate to distant tumors, eradicating also metastatic lesions. In contrast to systemic immunotherapy, the impact on immune cells irrelevant for the anti-tumor response is reduced. There are two approaches to tumor-directed immunotherapy: tumor-directed immunotherapy by administration route and tumor-directed immunotherapy by design. Tumor-directed immunotherapy by administration route is achieved by administering the immunomodulatory antibody directly into the tumor, into tumor-draining lymph nodes, or by a slow-release combination close to the tumor site. The immune stimulation is thereby focusing on the tumor area, minimizing systemic exposure and thus reducing systemic side effects. Tumor-directed immunotherapy by design can be achieved using bispecific cross-linking-dependent agonistic TNFR antibodies where a tumor-binding part mediates the cross-linking, replacing the need for FcγR-mediated cross-linking. In the absence of tumor cells, these types of bispecific antibodies will not be active, minimizing systemic immune activation and reducing systemic side effects
