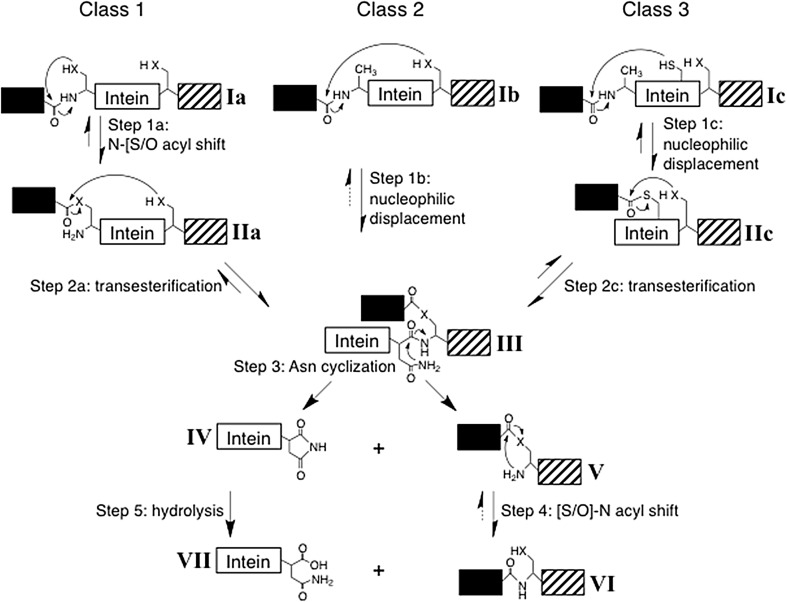
Fig. 1.
Intein-mediated protein splicing mechanisms. The majority of inteins follow the class 1 intein-mediated protein splicing mechanism, which consists of four coordinated nucleophilic displacements and requires Ser1, Thr1 or Cys1 as the intein N-terminal residue. Step 1a results in a linear (thio)ester intermediate and step 2a results in BIG with Cys+1, Ser+1 or Thr+1 as the branch point. Class 2 and 3 inteins do not require an intein N-terminal nucleophile. Class 2 inteins directly form BIG when the +1 residue attacks the N-terminal splice junction peptide bond. Class 3 inteins use a conserved Cys at Block F position 4 (CysF:4) to initiate protein splicing resulting in formation of the class-specific BIF. Once BIG is formed, the remaining reactions are the same for all inteins. The acyl shift in step 4 is rapid and spontaneous. Step 5 is also spontaneous, but is often slow. Solid arrows represent steps that have been experimentally verified while dashed arrows represent theoretical steps. Note that steps 1 and 2 are reversible; the forward reactions are driven by kinetic rates, equilibrium positions toward the forward reaction, and substrate/intermediate elimination as the protein moves toward the final products, among other factors. Intein residues and flanking extein residues that assist these reactions are not shown, nor are tetrahedral intermediates. ‘X’ represents the sulfur or oxygen atom in the side chain of Cys, Ser or Thr