Figure 2.
Domain Organization of BRPF1 and Its Variants from Individuals with Developmental Anomalies
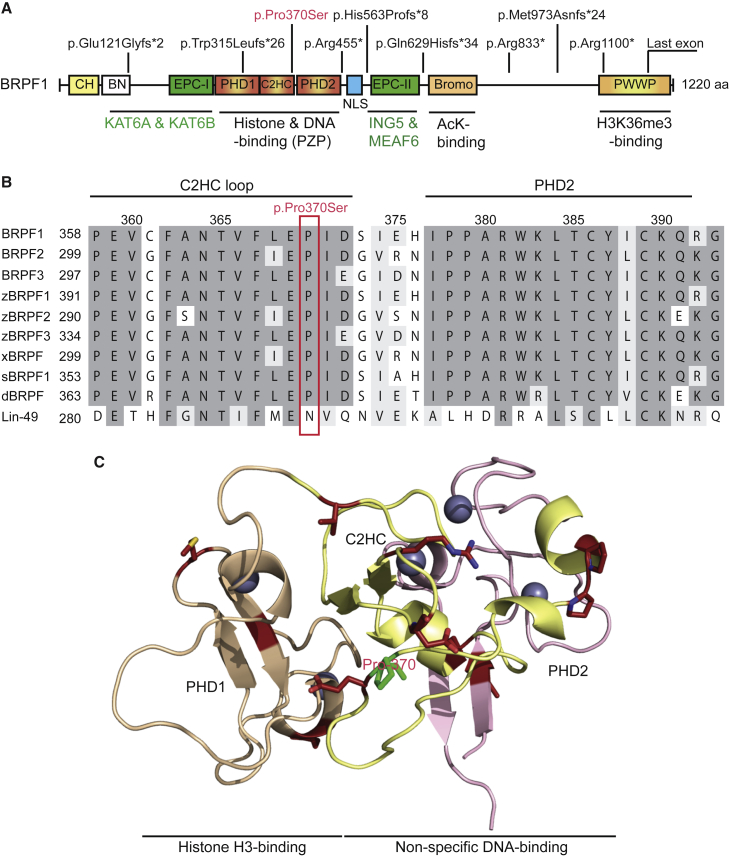
(A) Schematic representation of BRPF1 and nine variants as identified in ten individuals. See Table 1 for DNA sequence changes in the individuals. BRPF1 possesses multiple modules, including the PZP domain, bromodomain, and PWWP domain, for chromatin association. The PZP domain comprises two PHD fingers linked with a C2HC zinc finger. The first PHD finger recognizes the N terminus of histone H3.21, 23, 34 The C2HC zinc knuckle and the second PHD finger form a non-specific DNA binding domain.22, 23 The bromodomain has acetyllysine-binding ability,35 and the PWWP domain targets trimethylated histone H3.36, 37 The EPC-like motif C-terminal to the PZP domain is essential for formation of a stable trimeric complex with ING5 and MEAF6.19, 20, 21 Through the EPC-like motif N-terminal to the PZP domain and a conserved region further N-terminal to this motif, BRPF1 interacts with and activates KAT6A, KAT6B, and KAT7.19, 20, 21 Unlike p.Pro370Ser, the other eight variants contain C-terminal truncations due to nonsense or reading-frameshift mutations. These mutations are not located within the last coding exon and might trigger NMD in vivo. Abbreviations are as follows: CH, C2H2 zinc finger; BN, conserved BRPF N-terminal domain; EPC, enhancer of polycomb-like motif; NLS, nuclear localization signal.
(B) Sequence alignment of human BRPF1 with its paralogs (BRPF2 and BRPF3), as well as the orthologs from zebrafish (z), Xenopus (x), sea urchin (s), Drosophila (d), and C. elegans (Lin-49). There is one ortholog per organism from the worm to Xenopus, but there are three in zebrafish. Pro370 of BRPF1 and its corresponding residues in its paralogs and orthologs are boxed in red.
(C) Pro370 is located within a loop connecting the C2HC knuckle to the second PHD finger. PHD1 recognizes the free N terminus of histone H3,21, 23, 34 whereas the C2HC zinc knuckle and PHD2 form a module for non-specific interaction with the DNA backbone of the nucleosome.23 The structural model was generated based on a published report,23 with kind help of Tatiana G. Kutateladze.