Abstract
A total of 153 Burkholderia cepacia strains obtained from 153 French patients with cystic fibrosis were identified as Burkholderia multivorans (51.6%) or Burkholderia cenocepacia (45.1%). Eighty-two genotypes were identified using PvuII and EcoRI ribotyping. B. multivorans genotype A (found in 32 French patients) and two other genotypes were also identified among isolates from Austrian, German, Italian, and Canadian patients.
Burkholderia cepacia, originally described as a plant pathogen (10), is now a recognized cause of nosocomial infections (16). B. cepacia emerged in the 1980s as an important organism colonizing the lungs of patients with cystic fibrosis (CF) (18). B. cepacia acquisition has been associated with accelerated decline of lung function and, in some cases, with respiratory failure and death (16). It is difficult to eradicate pulmonary B. cepacia infections (6). Taxonomic studies have shown that the B. cepacia complex comprises at least nine closely related species, or genomovars (29). The genomovars are difficult to identify by phenotyping methods (8, 25, 31, 32). Identifying the species and genotype distribution within a given country could have important implications for infection control, clinical outcome, and studies of bacterial virulence and host susceptibility (13). Neither the epidemiology of B. cepacia complex nor its genomovar distribution has been studied in French CF patients.
The main purpose of this study was to determine the distribution of B. cepacia complex genomovars in French CF centers. As there are diverse strains in the genomovars, we used ribotyping to compare these isolates and sought a possible genomovar-ribotype correlation. Ribotyping has previously proved valuable for discriminating among B. cepacia complex isolates (4, 5, 9). We also compared the French isolates with strains from other countries.
A total of 153 B. cepacia complex isolates were obtained from 153 patients in 27 French CF centers between 1995 and 2000. These patients received routine care at their CF center, were infected with B. cepacia during the study period, and had not been infected previously (26). All the isolates studied were the first B. cepacia isolates cultured during this period and were sent to us for genomovar and species identification. Prior samples from these patients were negative for B. cepacia. Between 1 and 37 isolates were collected per center.
Isolates were first examined by whole-cell protein electrophoresis (23). Detailed taxonomic studies have demonstrated that this method is an appropriate tool to differentiate members of the B. cepacia complex from other Burkholderia species and biochemically similar bacteria (12, 14). However, these studies also revealed that strains from some B. cepacia complex species are misidentified (12, 13, 30). Therefore, all isolates were further identified using HaeIII and MnlI-recA restriction fragment length polymorphism (RFLP) (23).
Ribotyping was performed using a RiboPrinter (Qualicon, Wilmington, Del.) and the restriction enzymes EcoRI and PvuII by standard protocols (9). The PvuII ribotype patterns of the French CF isolates were compared with those of the 178 B. cepacia complex strains contained in the Genetic Epidemiology Network for Europe (GENE) database (www.ewi.med.uu.nl/gene), which were isolated from the environment (n = 32; 11 from Italy and 21 from the United Kingdom) or from CF patients (n = 124; 55 from Italy, 31 from The Netherlands, 33 from Germany, 4 from Canada, and 1 from Austria) or were taken from reference culture collections (n = 22). The ribotype patterns of two strains representative of the ET12 clone, identified by multilocus enzyme electrophoresis analysis (19) and shown to cause isolated infections and outbreaks both in Ontario, Canada, and the United Kingdom, and 20 reference strains from culture collections are also present in the GENE database (9).
The whole-cell protein profiles (not shown) and the fact that a recA amplicon of the expected size was obtained (23) confirmed that all 153 French isolates belonged to the B. cepacia complex. Seventy-nine (51.6%) of the isolates exhibited four different Burkholderia multivorans recA RFLP profiles, 69 (45.1%) exhibited five different Burkholderia cenocepacia recA RFLP profiles (genomovar III), two (1.3%) exhibited two different Burkholderia pyrrocinia recA RFLP profiles (genomovar IX), two (1.3%) exhibited one Burkholderia stabilis recA RFLP profile (genomovar IV), and one (0.6%) exhibited one Burkholderia vietnamiensis recA RFLP profile (genomovar V).
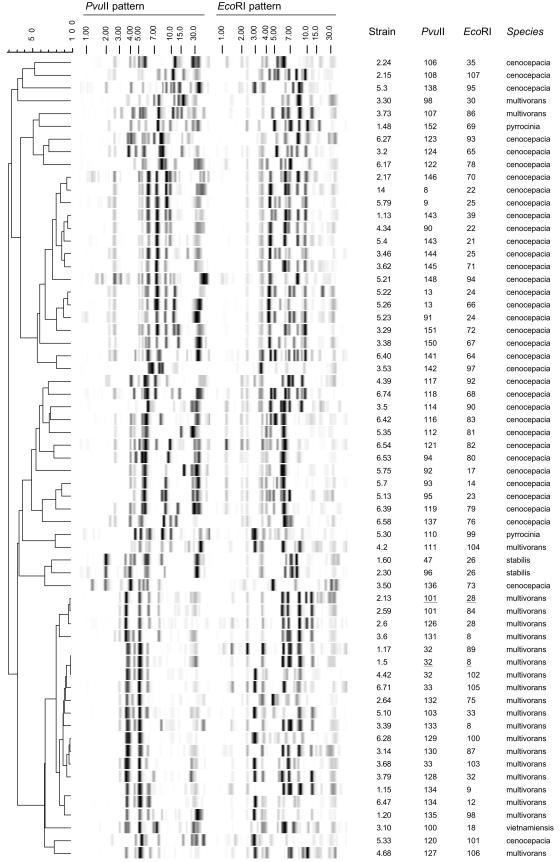
The 153 French B. cepacia isolates were ribotyped separately with PvuII and with EcoRI, yielding 62 and 59 ribotype patterns, respectively (Fig. 1). Combined analysis of the ribotype patterns obtained with the two enzymes yielded 82 genotypes. Two genotypes predominated: genotype A (PvuII-32/EcoRI-8; B. multivorans) was identified in 32 patients, and genotype B (PvuII-101/EcoRI-28; B. multivorans) was identified in 17 patients. The next most frequent genotype (PvuII-93/EcoRI-14; B. cenocepacia) was identified in eight patients.
FIG. 1.
Overview of distinct PvuII and EcoRI ribotype patterns. The dendrogram was obtained after unweighted pair group method with arithmetic mean clustering of PvuII ribotype patterns using Pearson correlation coefficients (which are transformed into percentage values on the scale). The two major B. multivorans genotypes, genotypes A (PvuII-32/EcoRI-8) and B (PvuII-101/EcoRI-28), are underlined. The molecular size scale (in kilobases) is shown above the patterns. The strain codes, PvuII and EcoRI ribotypes, and Burkholderia species are shown to the right of the patterns.
Each ribotype pattern, obtained with either EcoRI or PvuII, corresponded to a single species. The B. multivorans isolates yielded 23 EcoRI and 17 PvuII ribotypes, and the B. cenocepacia isolates yielded 44 EcoRI and 39 PvuII ribotypes. The two B. pyrrocinia isolates and the B. vietnamiensis isolate had distinct ribotypes with each enzyme, while the two B. stabilis isolates had the same EcoRI ribotype but distinct PvuII ribotypes. Combined analysis of the ribotype patterns obtained with the two enzymes yielded 26 genotypes for the 79 B. multivorans isolates and 51 genotypes for the 69 B. cenocepacia isolates. This is the first demonstration that combined use of these two enzymes improves strain discrimination in this setting.
We then compared the PvuII ribotype patterns of the 153 French isolates with those deposited in the GENE database, as ribotype patterns from different riboprinters are reproducible (7). None of the French isolates matched the ribotype patterns of the two ET12 strains, suggesting that the ET12 lineage is absent from French CF centers. Six PvuII ribotypes corresponded to both French CF isolates and GENE database isolates. Two of these ribotypes (PvuII-32 and PvuII-33) corresponded to B. multivorans, three (PvuII-8, PvuII-9, and PvuII-13) corresponded to B. cenocepacia, and one (PvuII-47) corresponded to B. stabilis. We then performed EcoRI ribotyping of the GENE database isolates. All the PvuII ribotypes showed EcoRI ribotype diversity, with the exception of B. stabilis PvuII-47. Three combined PvuII-EcoRI genotypes were found among both the French CF isolates and the GENE database isolates. Genotype A (B. multivorans PvuII-32/EcoRI-8) was found in 32 French CF patients and one Austrian CF patient. The B. multivorans PvuII-33/EcoRI-8 genotype was found in two French patients, four German patients, and one Canadian CF patient. The B. stabilis PvuII-47/EcoRI-26 genotype was found in one French patient, two German patients, and one Italian CF patient and in one German water-outlet isolate.
A small number of CF populations in different countries have been characterized in this respect. Our study of the B. cepacia complex species distribution in French CF centers shows that B. multivorans is the most prevalent (51%), followed by B. cenocepacia (45%). Similar results were obtained by others. Heath et al. (17) reported that B. multivorans (46%) was slightly more prevalent than B. cenocepacia (44%) in 56 American CF patients. Turton et al. (28) reported similar findings (B. multivorans [39%], B. cenocepacia [29%]) in a study of 111 British CF patients. The high percentage of B. multivorans observed in our study suggests interpatient transmission or acquisition from a common source, as only two genotypes were found among 49 CF patients harboring B. multivorans. In contrast, Heath et al. (17) found that most of their patients harbored strains with unique genotypes. Similarly, Mahenthiralingam et al. found no evidence of patient-to-patient spread of B. multivorans (24). Other studies have found a predominance of B. cenocepacia. LiPuma et al. (22), studying 606 patients in the United States, found B. cenocepacia in 50% of CF patients and B. multivorans in 38% of CF patients. Similar results were found in Portugal, with a B. cenocepacia prevalence of 52% (15) and in Australia with a prevalence of 39% (20). In contrast, B. cenocepacia is largely predominant in Canada (80%) (27), Italy (72 and 86%) (1, 3), and the United Kingdom (76%) (11). In some studies, other genomovars reach significant percentages, such as genomovar I (36%) and B. stabilis (18%) in Portugal (15) or genomovar I in Australia (29%) (20). Differences in species distribution in these studies seem to be due to specificities of the CF subpopulations examined.
We found that isolates belonging to different species always had different ribotypes, confirming the excellent capacity of this approach to distinguish among B. cepacia complex species (9). Ribotyping-based species identification of a given isolate can be achieved only if the corresponding ribotype pattern is already present in the database. However, this limitation can be overcome by cluster analysis, as the ribotype patterns obtained for a given species tend to resemble each other and to cluster into a single branch (Fig. 1).
We found that some genotypes, including the frequent genotype A, were present both in French CF patients and in GENE database isolates. These cases possibly correspond to independent acquisition by different patients from natural (environmental) sources in which these strains are highly prevalent (2, 21), but the precise importance of this mode of acquisition remains to be determined (21).
Acknowledgments
This work was supported in part by the patient association Vaincre la Mucoviscidose and by the European Union Fifth Framework Programme, concerted action grant QLK2-2000-01404 (Network for Automated Bacterial Strain Fingerprinting in Europe, GENE project).
We are indebted to J. Govan for providing environmental B. cepacia complex isolates, to S. Ravilly for advice, and to J. Navarro for helpful discussions. We thank the physicians and investigators from the following CF centers for providing us with the 153 B. cepacia isolates: Besançon, Brest, Cahors, Clermont-Ferrand, Dax, Paris (Cochin, Necker, Trousseau, and Robert-Debré), Dijon, Giens, Lille, Lyon, Metz, Nancy, Pessac, Rennes, Roscoff, Rouen, St. Denis (Réunion), St. Nazaire, St. Trojean, Strasbourg, Suresnes, Toulouse, Tours, and Versailles.
REFERENCES
- 1.Agodi, A., E. Mahenthiralingam, M. Barchitta, V. Giannino, A. Sciacca, and S. Stefani. 2001. Burkholderia cepacia complex infection in Italian patients with cystic fibrosis: prevalence, epidemiology, and genomovar status. J. Clin. Microbiol. 39:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balandreau, J., V. Viallard, B. Cournoyer, T. Coenye, S. Laevens, and P. Vandamme. 2001. Burkholderia cepacia genomovar III is a common plant-associated bacterium. Appl. Environ. Microbiol. 67:982-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevivino, A., C. Dalmastri, S. Tabacchioni, L. Chiarini, M. L. Belli, S. Piana, A. Materazzo, P. Vandamme, and G. Manno. 2002. Burkholderia cepacia complex bacteria from clinical and environmental sources in Italy: genomovar status and distribution of traits related to virulence and transmissibility. J. Clin. Microbiol. 40:846-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingen, E. H., E. Denamur, and J. Elion. 1994. Use of ribotyping in epidemiological surveillance of nosocomial outbreaks. Clin. Microbiol. Rev. 7:311-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingen, E. H., M. Weber, J. Derelle, N. Brahimi, N. Y. Lambert-Zechovsky, M. Vidailhet, J. Navarro, and J. Elion. 1993. Arbitrarily primed polymerase chain reaction as a rapid method to differentiate crossed from independent Pseudomonas cepacia infections in cystic fibrosis patients. J. Clin. Microbiol. 31:2589-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonacorsi, S., F. Fitoussi, S. Lhopital, and E. Bingen. 1999. Comparative in vitro activities of meropenem, imipenem, temocillin, piperacillin, and ceftazidime in combination with tobramycin, rifampin, or ciprofloxacin against Burkholderia cepacia isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 43:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisse, S., V. Fussing, B. Ridwan, J. Verhoef, and R. J. Willems. 2002. Automated ribotyping of vancomycin-resistant Enterococcus faecium isolates. J. Clin. Microbiol. 40:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisse, S., S. Stefani, J. Verhoef, A. Van Belkum, P. Vandamme, and W. Goessens. 2002. Comparative evaluation of the BD Phoenix and VITEK 2 automated instruments for identification of isolates of the Burkholderia cepacia complex. J. Clin. Microbiol. 40:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brisse, S., C. M. Verduin, D. Milatovic, A. Fluit, J. Verhoef, S. Laevens, P. Vandamme, B. Tummler, H. A. Verbrugh, and A. van Belkum. 2000. Distinguishing species of the Burkholderia cepacia complex and Burkholderia gladioli by automated ribotyping. J. Clin. Microbiol. 38:1876-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkholder, W. H. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115-117. [Google Scholar]
- 11.Clode, F. E., M. E. Kaufmann, H. Malnick, and T. L. Pitt. 2000. Distribution of genes encoding putative transmissibility factors among epidemic and nonepidemic strains of Burkholderia cepacia from cystic fibrosis patients in the United Kingdom. J. Clin. Microbiol. 38:1763-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coenye, T., J. Goris, T. Spilker, P. Vandamme, and J. J. LiPuma. 2002. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J. Clin. Microbiol. 40:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coenye, T., and J. J. LiPuma. 2003. Molecular epidemiology of Burkholderia species. Front. Biosci. 8:e55-67. [DOI] [PubMed] [Google Scholar]
- 14.Coenye, T., P. Vandamme, J. R. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha, M. V., J. H. Leitao, E. Mahenthiralingam, P. Vandamme, L. Lito, C. Barreto, M. J. Salgado, and I. Sa-Correia. 2003. Molecular analysis of Burkholderia cepacia complex isolates from a Portuguese cystic fibrosis center: a 7-year study. J. Clin. Microbiol. 41:4113-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govan, J. R., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 17.Heath, D. G., K. Hohneker, C. Carriker, K. Smith, J. Routh, J. J. LiPuma, R. M. Aris, D. Weber, and P. H. Gilligan. 2002. Six-year molecular analysis of Burkholderia cepacia complex isolates among cystic fibrosis patients at a referral center for lung transplantation. J. Clin. Microbiol. 40:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, W. M., S. D. Tyler, and K. R. Rozee. 1994. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J. Clin. Microbiol. 32:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidd, T. J., S. C. Bell, and C. Coulter. 2003. Genomovar diversity among Burkholderia cepacia complex isolates from an Australian adult cystic fibrosis unit. Eur. J. Clin. Microbiol. Infect. Dis. 22:434-437. [DOI] [PubMed] [Google Scholar]
- 21.LiPuma, J. J., T. Spilker, T. Coenye, and C. F. Gonzalez. 2002. An epidemic Burkholderia cepacia complex strain identified in soil. Lancet 359:2002-2003. [DOI] [PubMed] [Google Scholar]
- 22.LiPuma, J. J., T. Spilker, L. H. Gill, P. W. Campbell III, L. Liu, and E. Mahenthiralingam. 2001. Disproportionate distribution of Burkholderia cepacia complex species and transmissibility markers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 164:92-96. [DOI] [PubMed] [Google Scholar]
- 23.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 38:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahenthiralingam, E., P. Vandamme, M. E. Campbell, D. A. Henry, A. M. Gravelle, L. T. Wong, A. G. Davidson, P. G. Wilcox, B. Nakielna, and D. P. Speert. 2001. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin. Infect. Dis. 33:1469-1475. [DOI] [PubMed] [Google Scholar]
- 25.McMenamin, J. D., T. M. Zaccone, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Misidentification of Burkholderia cepacia in US cystic fibrosis treatment centers: an analysis of 1,051 recent sputum isolates. Chest 117:1661-1665. [DOI] [PubMed] [Google Scholar]
- 26.Segonds, C., E. Bingen, G. Couetdic, S. Mathy, N. Brahimi, N. Marty, P. Plesiat, Y. Michel-Briand, and G. Chabanon. 1997. Genotypic analysis of Burkholderia cepacia isolates from 13 French cystic fibrosis centers. J. Clin. Microbiol. 35:2055-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speert, D. P., D. Henry, P. Vandamme, M. Corey, and E. Mahenthiralingam. 2002. Epidemiology of Burkholderia cepacia complex in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 8:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turton, J. F., M. E. Kaufmann, N. Mustafa, S. Kawa, F. E. Clode, and T. L. Pitt. 2003. Molecular comparison of isolates of Burkholderia multivorans from patients with cystic fibrosis in the United Kingdom. J. Clin. Microbiol. 41:5750-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandamme, P., B. Holmes, T. Coenye, J. Goris, E. Mahenthiralingam, J. J. LiPuma, and J. R. Govan. 2003. Burkholderia cenocepacia sp. nov.—a new twist to an old story. Res. Microbiol. 154:91-96. [DOI] [PubMed] [Google Scholar]
- 30.Vandamme, P., E. Mahenthiralingam, B. Holmes, T. Coenye, B. Hoste, P. De Vos, D. Henry, and D. P. Speert. 2000. Identification and population structure of Burkholderia stabilis sp. nov. (formerly Burkholderia cepacia genomovar IV). J. Clin. Microbiol. 38:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Pelt, C., C. M. Verduin, W. H. Goessens, M. C. Vos, B. Tummler, C. Segonds, F. Reubsaet, H. Verbrugh, and A. van Belkum. 1999. Identification of Burkholderia spp. in the clinical microbiology laboratory: comparison of conventional and molecular methods. J. Clin. Microbiol. 37:2158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermis, K., P. A. Vandamme, and H. J. Nelis. 2003. Burkholderia cepacia complex genomovars: utilization of carbon sources, susceptibility to antimicrobial agents and growth on selective media. J. Appl. Microbiol. 95:1191-1199. [DOI] [PubMed] [Google Scholar]