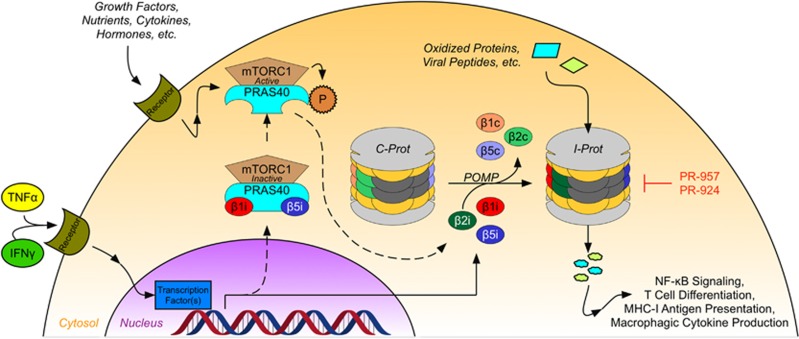
Figure 2.
The i-prot has distinct features compared with the constitutional proteasome. The i-prot is formed when the β1c, β2c and β5c subunits of the c-prot are replaced by β1i and β5i, respectively. The expression of these subunits is promoted – at least in part – by IFNγ and TNFα signaling. Once expressed, β1i and β5i associate with the chaperone protein POMP, which facilitates their incorporation into the proteasome. In AML, however, it has been demonstrated that β1i and β5i are sequestered by PRAS40, a component of mTORC1. Once mTORC1 is activated by any of a number of methods (hyperactivation of mTORC1 is a feature of numerous malignancies), it phosphorylates PRAS40 and releases β1i and β5i, thus facilitating the assembly of the i-prot. The incorporation of these different subunits alters the proteolytic substrate selectivity of the i-prot, granting the i-prot distinct functions as compared with the c-prot.