Abstract
Introduction
Dental pulp regeneration is a part of regenerative endodontics, which includes isolation, propagation, and re-transplantation of stem cells inside the prepared root canal space. The formation of new blood vessels through angiogenesis is mandatory to increase the survival rate of re-transplanted tissues. Angiogenesis is defined as the formation of new blood vessels from preexisting capillaries, which has great importance in pulp regeneration and homeostasis. Here the contribution of human dental pulp stem cells and proangiogenic and antiangiogenic factors to angiogenesis process and regeneration of dental pulp is reviewed.
Methods
A search was performed on the role of angiogenesis in dental pulp regeneration from January 2005 through April 2014. The recent aspects of the relationship between angiogenesis, human dental pulp stem cells, and proangiogenic and antiangiogenic factors in regeneration of dental pulp were assessed.
Results
Many studies have indicated an intimate relationship between angiogenesis and dental pulp regeneration. The contribution of stem cells and mechanical and chemical factors to dental pulp regeneration has been previously discussed.
Conclusions
Angiogenesis is an indispensable process during dental pulp regeneration. The survival of inflamed vital pulp and engineered transplanted pulp tissue are closely linked to the process of angiogenesis at sites of application. However, the detailed regulatory mechanisms involved in initiation and progression of angiogenesis in pulp tissue require investigation.
Keywords: Angiogenesis, proangiogenic and antiangiogenic factors, pulp regeneration, pulp stem cells
In blood vessel formation, the terms vasculogenesis and angiogenesis have been distinctively discussed. Vasculogenesis is defined as the formation of the primary vascular plexus from preexisting vascular precursor cells in the embryo (1). However, angiogenesis is the formation of new blood vessel from preexisting capillaries (1) and is responsible for the majority of the blood vessels formed during physiological and pathologic conditions (2, 3). Angiogenesis is initiated as a result of insufficient oxygen and nutrient supply and is regulated by tightly balanced production of numerous stimulatory and inhibitory chemobiological molecules such as growth factors, cytokines, matrix metalloproteinases (MMPs), endogenous angiogenesis inhibitors, transcription factors, adhesion molecules, and also components of the extracellular matrix (ECM) (4–8). The therapeutic modulation of angiogenesis process includes antiangiogenic therapies for fighting against malignancies (9–13) and proangiogenic therapies in repairing cardiovascular diseases and wound healing disorders by new blood vessels supplying blood to damaged tissues (9, 10, 14).
Human dental pulp is a highly vascularized tissue, which because of its vascular network and progenitor or postnatal dental pulp stem cells (DPSCs) has an impressive naturally inherent regenerative capacity (15–17). Dental pulp regeneration is part of the regenerative endodontic concept, which provides replacements for damaged tooth structures including pulp-dentin complex (18). It is a field in regenerative medicine and a branch of tissue engineering, which uses stem cells, biochemical factors, and engineering materials to replace lost or impaired biological tissues (19, 20). After isolation, the tissue-engineered stem cells are propagated in special medium and transplanted inside the prepared root canal space to develop into new pulp tissue (18). The success of tissue engineering depends on oxygen and nutrient transport to the implanted cells. If blood supply cannot be established rapidly, necrosis of the transplant will occur (21). This rule is also applicable to dental pulp regeneration, where angiogenesis is a key to both the development and regeneration of the dentin-pulp complex (17, 22). Angiogenesis establishes the blood supply and brings the oxygen, nutrition, and prevascular stem cells for regeneration (23).
Here we discuss an overview of the role of angiogenesis in dental pulp regeneration and the proangiogenic or antiangiogenic factors involved. The main aspects pursued in this review include the following:
The evaluation of the current state and the research trend regarding the role of angiogenesis in regenerative endodontics from January 2005 through April 2014.
The determination of the elements or components, such as the stem cells or proangiogenic or antiangiogenic factors, that are involved directly in the angiogenesis process in dental pulp regeneration and the field of regenerative endodontics.
The clarification of strong and weak points regarding the angiogenesis events in dental pulp regeneration to introduce the present challenges and complexities in regenerative endodontic procedures that should be taken into consideration in research studies. Regarding the current state, in the conclusion section for each evaluated heading, the current state and research trends have been introduced and also the weak points or challenges are addressed to aid future studies to target these points.
Materials and Methods
Purpose of Review
The present review was conducted to evaluate the role of angiogenesis in dental pulp regeneration. Specifically, the potential effects of cell-related factors such as the contribution of stem cells and the proangiogenic and antiangiogenic factors in dental pulp regeneration were reviewed through the literature.
Inclusion and Exclusion Criteria
The inclusion criteria considered all articles including review studies, in vitro or in vivo studies, and case reports in peer-reviewed journals published in English from January 2005 through April 2014 that evaluated the cellular and elemental factors that are directly related to angiogenesis process in dental pulp regeneration. The studies that investigated the effects of angiogenic factors in regenerative endodontic procedures were included, whereas other investigations that did not address these criteria were excluded.
Search Methodology
The search methodology used in this review article included electronic searches that were done in the PubMed database by using key words mentioned in the MeSH headings regarding the role of angiogenesis in the dental pulp regeneration and the stimulatory or inhibitory factors in pulpal angiogenesis process.
Search Strategy
In the electronic search of scientific papers in the PubMed database the following key words were used in combination with angiogenesis: dental pulp regeneration, dental pulp stem cells, orthodontic forces, proangiogenic growth factors such as vascular endothelial growth factor, fibroblast growth factor, platelet-derived growth factor, angiopoietins, matrix metalloproteinase, stem cell factor, and antiangiogenic growth factors. A number of full-text articles and the reference lists of the relevant articles were also evaluated to supplement the search. The evaluation of the eligibility and finding of relevant data was done independently by 2 reviewers. A third reviewer was selected for resolving of any disagreements met during evaluation processes.
Dental Pulp Regeneration
Contributions of DPSCs and Angiogenesis
In stem cell biology, which is a part of tissue regenerative procedures, the highly potential stem cells, which are provided from vital tissues, are propagated and used for treatment of different pathologic conditions. Among different sources for stem cells such as umbilical cord blood, bone marrow, peripheral blood, and adipose tissue, the most common sources of stem cells used in this field are the mesenchymal stem cells isolated from bone marrow (BM-MSCs) (24, 25). In addition, DPSCs are introduced as another source for tissue regeneration procedures (18, 24, 25). DPSCs are postnatal, multipotent stem cells with similarities and some limitations to BM-MSCs (18, 26). Several authors have also reported some advantages in clinical usage of DPSCs including lower mortality rate, less legal or ethical issues, easy access from extracted teeth, and cryopreservation without losing their multi-differentiation potential (24–28).
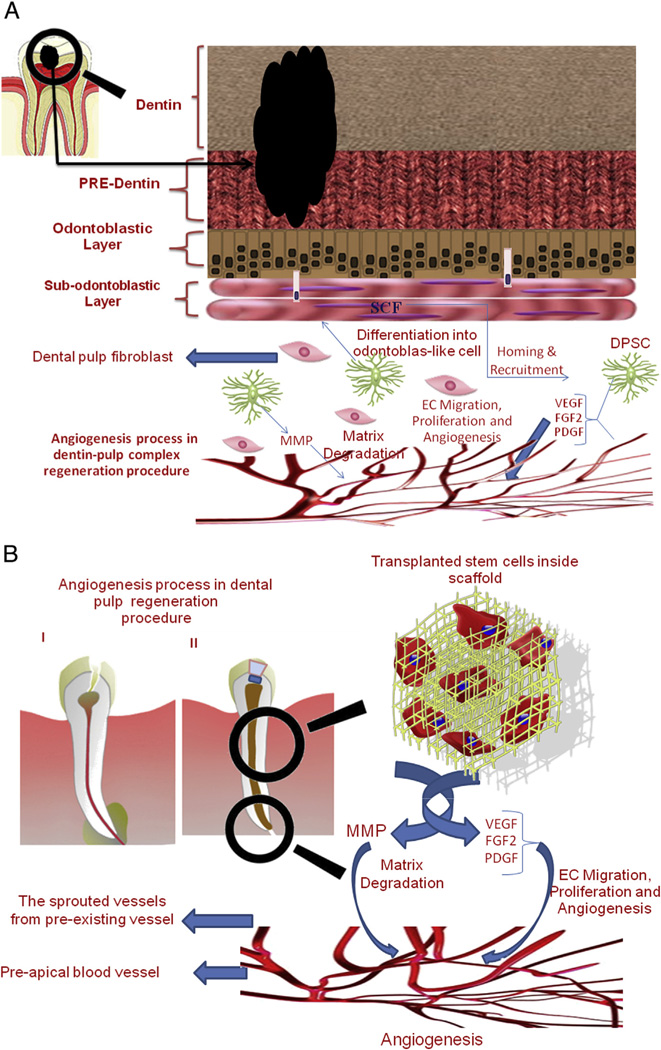
Sieveking and Ng (29) found 2 distinct roles for stem cells such as BM-MSCs. They may act in a paracrine fashion through expression of proangiogenic factors. Alternatively, they may differentiate into endothelial cells and directly participate in neoangiogenesis. The activity of human dental pulp cells in secretion of proangiogenic factors has been well-documented by many authors (30–35). Bronckaers et al (30) demonstrated that human dental pulp stem cells (hDPSCs) are able to induce angiogenesis in a paracrine fashion through expression of a wide range of angiogenic factors including vascular endothelial growth factor (VEGF) and monocyte cheomtactic protein-1 (MCP-1). Furthermore, hDPSCs can stimulate the migration of endothelial cells through activation of the PI3K/AKT and MEK/ERK signaling pathways in vitro. Other authors have also shown that DPSCs are able to secrete a variety of proangiogenic factors including VEGF, fibroblast growth factor 2 (FGF-2), platelet-derived growth factor (PDGF), insulin-like growth factor 1 (IGF-1), MMP-9, and transforming growth factor beta (TGF-β) and promote the migration and tubulogenesis activity of endothelial cells (22, 31–35) (Fig. 1).
Figure 1.
Schematic presentation of angiogenesis process in regenerative endodontic procedures: (A) the dentin-pulp complex regenerative process including the contributions of DPSCs and growth factors contribution in the angiogenesis process occurring through this regenerative process; (B) the dental pulp regeneration procedure performed inside a prepared root canal by transplanting a tissue-engineered scaffold including stem cells, which secret the necessary growth factors for sprouting angiogenesis.
Concerning the endothelial differentiation potential of hDPSCs, several investigators have indicated that in the presence of specialized differentiation medium, hDPSCs can express some of the endothelial cell markers including CD31, CD105, CD34, and von Willebrand factor in vitro (36–38). Janebodin et al (39) indicated that DPSCs can resemble perivascular supporting cells and induce more mature blood vessels when co-cultured with endothelial cells.
The dental pulp stem cells of exfoliated teeth (SHED) are capable of differentiating into endothelial-like cells (40, 41). Bento et al (41) indicated that VEGF/MEK1/ERK signaling pathway is a key regulator of the endothelial differentiation of DPSCs. Liu et al (42) showed that the inhibition of miR-424 might assist the dental pulp regeneration process. Kim et al (43) reported that a sudden increase in SIRT1 gene expression can up-regulate the angiogenic markers such as VEGF, FGF-2, and endothelial cell adhesion molecules such as vascular endothelial cadherin and platelet endothelial cell adhesion molecule-1. Dissanayaka et al (44) reported that the direct co-culture of DPSCs and endothelial cells can enhance the expression of angiogenic phenotype in vitro. Nakashima and Iohara (45) found that CD31(−)/CD146(−) or CD105(+) cell types isolated from dental pulp, in the presence of stromal cell–derived factor-1, can produce pulp tissue including vascular and neuronal structure in 14 days, formation of dentin in 35 days, and impose trophic action on endothelial cells. Other cells of pulp tissue seem to have remarkable effects on angiogenesis process. Tran-Hung et al (22) co-cultured dental pulp fibroblasts with human umbilical vein endothelial cells and indicated that fibroblasts can induce angiogenesis through secretion of FGF-2 and VEGF (Table 1).
TABLE 1.
Angiogenic Factors Involved in Dental Pulp Regeneration
| Type of element | Angiogenic effects | Mechanisms of action |
| DPSCs | Proangiogenic and antiangiogenic |
1. Expression of proangiogenic factors such as VEGF, FGF-2, PDGF, IGF-1, MMP-9, TGF-β, and MCP-1 |
| 2. Differentiation into endothelial-like cells | ||
| 3. Promoting endothelial cell migration by PI3K/AKT and MEK/ERK | ||
| Expression of antiangiogenic factors such as endostatin, IGFBP3, uPA, TIMP-1, and PAI-1 |
||
| SHED | Proangiogenic | Differentiating into endothelial-like cells through the VEGF/MEK1/ERK signaling pathway |
| Dental pulp fibroblasts | Proangiogenic | Inducing the secretion of FGF-2 and VEGF |
| Orthodontic movements | Proangiogenic | Expression of proangiogenic factors such as VEGF, FGF-2, PDGF, EGF, and TGF-β |
| Dentin matrix components | Proangiogenic and antiangiogenic |
In low concentrations act as a reservoir of proangiogenic factors |
| In high concentrations act as antiangiogenic agent due to unknown mechanism | ||
| VEGF | Proangiogenic | 1. Proliferation and migration of endothelial cells and maturation of sprouted capillary vessels |
| 2. Activation of intrinsic tyrosine kinases | ||
| 3. Differentiation of DPSCs into endothelial cells | ||
| FGF (FGF-1 and FGF-2) | Proangiogenic | Proliferation and migration of endothelial cells |
| PDGF | Proangiogenic | Proliferation and migration of perivascular supporting cells |
| Angiopoietins (Ang-1 and Ang-2) |
Proangiogenic | Initiating and stabilization of angiogenesis through competitive interactions with Tie-2 receptor |
| MMP | Proangiogenic | Degradation of ECM of vessel walls allowing migration of endothelial cells |
| SCF | Proangiogenic | Homing or navigation of stem cells |
| Iloprost | Proangiogenic | Expression of VEGF, FGF-2, and PDGF |
| Simvastatin | Proangiogenic | 1. Enhancing the endothelial cell functions |
| 2. Stimulation of DPSCs to express odontogenic and angiogenic markers | ||
| Hinokitiol | Proangiogenic | Production of hypoxia-inducible factor-1alpha and VEGF in pulp cells |
| L-mimosine | Proangiogenic | Expression of VEGF |
| LL-37 peptide | Proangiogenic | Migration of human pulp cells |
| Metallothionein | Proangiogenic | Enhancement of angiogenesis events and proliferation of differentiated odontoblast cells |
| Hyperglycemia | Antiangiogenic | Interfering with immune system cells function and inhibiting cellular proliferation |
Proangiogenic and Antiangiogenic Factors
Mechanical Stimulation
Muscle contractions promote angiogenesis through enhanced production of nitric oxide from vasodilated blood vessels (9). Higher capillary shear stress also increases the expression of VEGF and angiogenesis in skeletal muscles (46). Derringer et al (47,48) detected an increase in the number of microvessels in pulp tissue of orthodontically moved teeth that was due to the elevation of the angiogenic growth factors. They later used a combination of 5 neutralizing antibodies against VEGF, FGF-2, PDGF, epidermal growth factor (EGF), and TGF-β to assess the angiogenesis process in human dental pulp of orthodontically moved teeth (49). They observed a significant decrease in angiogenesis of pulp tissue of subjected teeth (49,50). Grünheid et al (51) studied the dental pulp cellular responses to orthodontic forces induced by elastic bands for 1–168 hours and reported a series of responses including macrophage invasion, cell proliferation, and angiogenesis during experimental monitoring. In 2007 Derringer and Linden (52)demonstrated the release of proangiogenic growth factor, EGF, in human dental pulp of orthodontically moved teeth. Thus, mechanical alteration in tooth has a significant impact on local angiogenesis and tissue regeneration.
Biochemical Stimulation
The biochemical stimulation of angiogenesis is related to the production of proangiogenic and antiangiogenic factors including growth factors. The growth factors such as bone morphogenetic proteins have an impeccable role in tissue engineering (53). The DPSCs can secrete proangiogenic factors such as VEGF, FGF-2, PDGF, MMP-9, IGF-1, TGF-β, interleukin-8, and MCP-1 (30). Roberts-Clark and Smith (54) acclaimed that dentin matrix can act as a reservoir for these angiogenic growth factors, and after any injury to pulp-dentin complex, these substances are released to promote angiogenesis in regenerating pulp tissue (Table 1). However, the molecular and cellular mechanisms involved remain poorly understood.
VEGF
VEGF is one of the major proangiogenic growth factors with many regulatory impacts on neuronal and vascular cell function such as survival, proliferation, migration, and sprouting of capillary vessels at the secreted sites (55–57). After binding to their respective receptors on the cell surface, VEGF and FGF can activate intrinsic tyrosine kinases and initiate signaling cascades that impact angiogenesis events (58). Three growth factors including VEGF, FGF, and angiopoietin-2 are mostly expressed as a result of hypoxia occurring in ischemic tissues or rigidly growing tumorigenic tissues (59). The exposure of hDPSCs and human dental pulp fibroblasts to hypoxic conditions resulted in an increase in the level of transcription factor hypoxia-inducible factor-1alpha and VEGF in pulp cells; however, the FGF level was not responsive to hypoxia (33) (Fig. 1A).
Among all proangiogenic factors, VEGF is the most essential factor for differentiation of vascular system (60). VEGF was shown to be a potent mitogen for endothelial cells, which can promote their proliferation and migration (61). The treatment of dental pulp tissue with 0–50 ng/mL recombinant human VEGF or recombinant human FGF-2 for 7 days resulted in increased number of microvessels and neovascularization (62). VEGF-A is a member of VEGF family that is more extensively studied (63). Other VEGF family members include VEGF-B, -C, and -D, which are also expressed in the dental pulp tissue and have autocrine and paracrine effects during angiogenesis (63).
Bronckaers et al (30) and others reported a very high amount of VEGF secretion by hDPSCs in dental pulp (22,32–35). Bento et al (41) introduced a key role for VEGF in differentiation of pulp stem cells into endothelial cells. The dental fibroblasts can also enhance angiogenesis by secretion of FGF-2 and VEGF (22). Güven et al (64) introduced an alternative pathway known as cyclooxygenase-2–dependent pathway for expression of VEGF protein in dental pulp. Other adjunct treatments might affect the secretion of VEGF protein. Scheven et al (65) used low-power ultrasound (30 kHz) on the odontoblast-like cells and suggested that ultrasound has an autocrine effect that can enhance the dentin repair by increasing the production of main VEGF-A isoforms including VEGF-120, VEGF-164, and to a lower amount VEGF-188. Thus, VEGF is a key regulator of angiogenesis in the pulp tissue whose alterations may have significant impact on maintenance and regulation of pulp tissue and tooth integrity (Table 1).
FGF
The FGF family members are mostly single-chain polypeptides with 2 prototypes and 22 members (12). The first prototype FGF-1, also known as acidic FGF, is the most important in the FGF family because it has the widest range of action and the capability of binding to all 7 FGF-receptor subtypes (12). FGF-1 can stimulate the proliferation and differentiation of all cell types required for blood vessel formation including endothelial cells (66–68). The other prototype FGF family member is FGF-2, also known as basic FGF, which is less potent than FGF-1and more potent than VEGF or PDGF. This prototype can enhance the proliferation of endothelial cells and also their organization into capillary-like network in vitro (66–68).
FGF proangiogenic factors are also well-discussed in the dental pulp regeneration processes (22, 30, 43). However, the majority of these studies were focused on the secretion and the function of FGF-2 molecule. As previously mentioned, FGF-2 can be produced by hDPSCs along with other proangiogenic factors (30) (Fig. 1A). This important growth factor is also produced by dental pulp fibroblasts (22). Kim et al (43) reported that a transient expression of SIRT1 gene can up-regulate the proangiogenic markers like FGF-2. Takeuchi et al (69) indicated that FGF-2, similar to granulocyte-colony stimulating factor, has wide effects on cell proliferation and migration during angiogenesis process. Li and Sae-Lim (70) demonstrated that FGF-1 applied on a collagen matrix carrier could induce the dental hard tissue formation like the dentin produced by Ca(OH)2 (Table 1).
PDGF
PDGF is a dimeric glycoprotein that is composed of 2 A (−AA) or 2 B (BB) chains or a combination of A and B (AB) and has the potential to induce cell proliferation (71). Keck et al (72) demonstrated that VEGF and PDGF-B share significant homology. Although both have mitogenic activity, they have different target cells and activities during angiogenesis events. The distinctive action of PDGF has also been mentioned by Matsuoka and Grotendorst (73). Generally, production of PDGF-BB by endothelial cells promotes the proliferation and migration of perivascular supporting cells to newly forming blood vessels. The interactions of perivascular supporting cells with endothelial cells through both paracrine and autocrine mechanisms are essential for stability and functionality of blood vessels.
The hDPSCs express this growth factor as well (22, 30,33–35) (Fig. 1A). Roberts-Clark and Smith demonstrated that the dentin matrix contains a higher amount of PDGF than other growth factors including VEGF and FGF-2 (54). Tran-Hung et al (32) showed that after dental pulp injury, production of PDGF-AB by endothelial cells is critical for recruitment of perivascular supporting cells to newly forming vessels and stabilization and maturation of these vessels.
Angiopoietins
The angiopoietins (Ang) are group of proteins with remarkable role in angiogenesis and lymphangiogenesis (74). Angiopoietins have 2 major forms including Ang-1 and Ang-2, which interact with Tie-1 and Tie-2 receptors on endothelial cells (74). Dental fibroblasts express Ang-2 along with other growth factors such as VEGF, FGF-2, PDGF, angiogenin, and EGF (22,75). El Karim et al (75) reported that different types of neuropeptides including calcitonin gene-related peptide, neuropeptide Y, substance P, and vasoactive intestinal polypeptide can modify the expression of mentioned angiogenic growth factors secreted from pulp fibroblasts. These factors play a critical role in initiation and stabilization of angiogenesis through competitive interactions with Tie-2 receptor (75). However, little is known about Tie-1 activity and functions during angiogenesis.
MMPs
MMPs are a group of important enzymes in angiogenesis. These biological molecules have a key role in degradation of the ECM of vessel walls, allowing the migration of endothelial cells (76) (Fig. 1). In 2009 Zheng et al (77) showed a significant increase in the level of MMP-3 and MMP-9 produced by either endothelial cells or endothelial progenitor cells in response to dental pulp injury. Muromachi et al (78) indicated that the amount of MMP-3 was increased after pulpotomy, which can induce the production of connective tissue growth factor/CCN family 2 (CTGF/CCN2) independent of the MMP-3 protease function. MMP-3 can dependently act on dynamin-related endocytosis and affects migration of pulp cells (78). In 2013 Ozeki et al (79) acclaimed that the proinflammatory cytokines including interleukin-1β, tumor necrosis factor-α, and interferon-γ can induce the production of MMP-3, increase cell proliferation, and present antiapoptosis effects on odontoblast-like cells derived from embryonic stem cells.
Stem Cell Factor
Stem cell factor (SCF) is a chemokine that is fast acting and plays a key role in hematopoiesis during embryonic period (80). It was shown that SCF is one of the important factors related to the migration of stem cells through the endothelium to be recruited in required site. In the process of recruitment of stem cells, the first step is the homing or navigation of these cells to the required site for repopulation and differentiation (81). Pan et al (82) evaluated the effect of this proangiogenic factor in regeneration of dental pulp and showed that high amounts of SCF can be found in sub-odontoblastic layer of Höhl in dental pulp (Fig. 1A). They reported that SCF has the potential to induce cell homing, angiogenesis, and tissue remodeling effects at applied sites (82).
Other Biochemical Substances
Limjeerajarus et al (83) showed that iloprost, a PGI2 analogue, can increase the expression of VEGF, FGF-2, and PDGF growth factors in pulp tissue (84). Simvastatin (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor) is a chemical substance from the statin family that can enhance angiogenesis and endothelial cell functions (85) and stimulate new bone formation. Min et al (86) assessed the effect of simvastatin on hDPSCs and found that after exposure to simvastatin, pulp stem cells can express odontogenic and angiogenic markers. Kim et al (87) used hinokitiol, a natural iron-chelating agent, on dental pulp cells and reported an increase in production of hypoxia-inducible factor-1alpha and VEGF in pulp cells and promotion of their angiogenic potential.
L-mimosine (prolyl hydroxylase inhibitor) can increase the expression of VEGF in applied tooth slices (88). Kajiya et al (89) acclaimed that LL-37 peptide can induce the migration of the human pulp cells and promote pulp-dentin complex regeneration. Izumi et al (90) localized metallothionein, a cysteine-rich protein, in dental pulp after injury and suggested that metallothionein is closely related to the angiogenesis events and proliferation of differentiated odontoblast cells during the healing process.
Biochemical Inhibitors
Bronckaers et al (30) reported that DPSCs can produce antiangiogenic factors including endostatin, IGFBP3, uPA, TIMP-1, and PAI-1. Roberts-Clark and Smith (54) indicated that the dentin matrix components in low concentrations have proangiogenic impact, whereas in high concentrations they have inhibitory effects on angiogenesis events of dental pulp. Hyperglycemia that is due to negative influence on immune system function can interfere with inflammatory phase including microvascular problems leading to chronic periodontal disease (91–94). Garber et al (95) reported no complete dentin bridge formation under pulp-capping material in diabetic rats. Hyperglycemia can also drastically affect inflammatory condition of dental pulp tissue and interfere with cellular proliferation and angiogenesis, which jeopardizes the pulp tissue healing. There is now a large family of endogenous inhibitors of angiogenesis whose function in tooth development and regeneration needs evaluation. These include thrombospondin-1 and thrombospondin-2, whose expression plays a significant role in vascular homeostasis and pathologic neovascularization. Another important physiologic antiangiogenic agent is platelet factor 4. Fragments of platelet factor 4 are effective inhibitors of angiogenesis process and are useful agents for therapeutic purposes (96). However, the antiangiogenic role of these agents on angiogenesis events of dental pulp tissue has yet to be discussed in studies.
Conclusions
Angiogenesis is an undeniable process during whole dental pulp regeneration. The survival of engineered and transplanted pulp tissue is closely related to formation of new blood vessels at applied sites. The role of angiogenesis in vital pulp therapies is also of utmost importance in this field. The neovascularization induced by hDPSCs, side population cells, and dental pulp fibroblasts in inflamed pulp tissue can promote the healing process of injured tissue. The contribution of DPSCs to angiogenesis has been more evaluated in paracrine secretion of a wide range of angiogenic factors. These cells have perivascular supporting cell topography and function and can promote the migration and tubulogenesis of endothelial cells. Although there is limited evidence regarding the direct differentiation of DPSCs to endothelial cells, the direct differentiation of DPSCs to endothelial cells is absolutely required for dental pulp regeneration procedures in closed apex teeth. The need for direct differentiation of hDPSCs to endothelial cells can be more complex and difficult to achieve where the pulp tissue transplantation is performed in dental canals prepared in matured teeth. In this situation, the closed apex is the major concern for formation of anastomosis between newly formed vessels in the transplanted tissue and periapical vascular network.
Mechanical stimulations by orthodontic forces appear to have positive effect on angiogenesis process. This positive effect is due to the increased expression of several growth factors such as VEGF, FGF2, PDGF, EGF, and TGF-β in dental pulp tissue. However, the exact mechanisms and the cells involved are not clearly defined and may be unique to dental pulp cells. The impact of chemical stimulation and inhibition of angiogenesis events is through the expression of many growth factors, which are well-discussed by others. However, these growth factors act as a double-edged sword in reality. It is clear that angiogenesis events are tightly orchestrated and regulated by several intrinsic and extrinsic factors. Hence, the imposed extrinsic chemical stimulatory or inhibitory factors should be used firmly under control of clinicians. We still lack detailed knowledge of the regulatory angiogenesis events in the dental pulp and balanced usage of growth factors. In future studies, cautious and controllable manipulation of growth factors should be treated as an important goal to establish a practical, functional, and nonhazardous regenerated pulp tissue with regulated and controlled neovascularization process at desired sites.
Acknowledgments
This article is dedicated to late Professor Kamal Asgar from the Department of Dental and Biological Materials at the University of Michigan (1922–2012) who was recently selected for Hall of Honor of School of Dentistry, Alumni Society Board of Governors, University of Michigan.
Special thanks to Ms Neda Bayati for artwork.
Footnotes
The authors deny any conflicts of interest related to this study.
References
- 1.Risau W, Flamme I. Vasculogenesis. Ann Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 2.Flamme I, Frölich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. 1997;173:206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Behzadian MA, Bartoli AB, El-Remessy B, et al. Cellular and molecular mechanisms of retinal angiogenesis. In: Penn JS, editor. Retinal and Choroidal Angiogenesis. New York: Springer; 2008. p. 119. [Google Scholar]
- 4.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhadada SV, Goyal BR, Patel MM. Angiogenic targets for potential disorders. Fundam Clin Pharmacol. 2011;25:29–47. doi: 10.1111/j.1472-8206.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Is angiogenesis an organizing principle in biology and medicine? J Pediatr Surg. 2007;42:1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 8.Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 9.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard D. Endothelial integrins and angiogenesis: not so simple anymore. J Clin Invest. 2002;110:913–914. doi: 10.1172/JCI16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stegmann TJ, Hoppert T, Schneider A, et al. Induction of myocardial neoangiogenesis by human growth factors: a new therapeutic approach in coronary heart disease. Herz. 2000;25:589–599. doi: 10.1007/pl00001972. (in German) [DOI] [PubMed] [Google Scholar]
- 12.Stegmann TJ. FGF-1: a human growth factor in the induction of neoangiogenesis. Expert Opin Investig Drugs. 1998;7:2011–2015. doi: 10.1517/13543784.7.12.2011. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Angiogenic therapy of the human heart. Circulation. 1998;97:628–629. doi: 10.1161/01.cir.97.7.628. [DOI] [PubMed] [Google Scholar]
- 14.Wagoner LE, Merrill W, Jacobs J, et al. Angiogenesis protein therapy with human fibroblast growth factor (FGF-1) results of a phase I open label, dose escalation study in subjects with CAD not eligible for PCI or CABG. Circulation. 2007;116:443. [Google Scholar]
- 15.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.About I, Bottero MJ, de Denato P, et al. Human dentin production in vitro. Exp Cell Res. 2000;258:33–41. doi: 10.1006/excr.2000.4909. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 18.Murray PE, Garcia-Godoy F, Hargreaves K. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 20.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 21.Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–2104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 22.Tran-Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. J Dent Res. 2006;85:819–823. doi: 10.1177/154405910608500908. [DOI] [PubMed] [Google Scholar]
- 23.Saghiri MA, Asatourian A, Sheibani N. Angiogenesis in regenerative dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:122. doi: 10.1016/j.oooo.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papaccio G, Graziano A, d’Aquino R, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–325. doi: 10.1002/jcp.20667. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Walboomers XF, Shi S, et al. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 26.Krebsbach PH, Robey PG. Dental and skeletal stem cells: potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002;66:766–773. [PubMed] [Google Scholar]
- 27.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 28.Nakashima M, Iohara K, Ishikawa M, et al. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11) Hum Gene Ther. 2004;15:1045–1053. doi: 10.1089/hum.2004.15.1045. [DOI] [PubMed] [Google Scholar]
- 29.Sieveking DP, Ng MK. Cell therapies for therapeutic angiogenesis: back to the bench. Vasc Med. 2009;14:153–166. doi: 10.1177/1358863X08098698. [DOI] [PubMed] [Google Scholar]
- 30.Bronckaers A, Hilkens P, Fanton Y, et al. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilkens P, Fanton Y, Martens W, et al. Pro-angiogenic impact of dental stem cells in vitro and vivo. Stem Cell Res. 2014;12:778–790. doi: 10.1016/j.scr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Tran-Hung L, Laurent P, Camps J, About I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch Oral Biol. 2008;53:9–13. doi: 10.1016/j.archoralbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Aranha AM, Zhang Z, Neiva KG, et al. Hypoxia enhances the angiogenic potential of human dental pulp cells. J Endod. 2010;36:1633–1637. doi: 10.1016/j.joen.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Matsushita K, Motani R, Sakuta T, et al. The role of vascular endothelial growth factor in human dental pulp cells: induction of chemotaxis, proliferation, and differentiation and activation of the AP-1-dependent signaling pathway. J Dent Res. 2000;79:1596–1603. doi: 10.1177/00220345000790081201. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20:435–440. doi: 10.1016/j.cytogfr.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 36.d’Aquino R, Graziano A, Sampaolesi M, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 37.Karbanova J, Soukup T, Suchanek J, et al. Characterization of dental pulp stem cells from impacted third molars cultured in low serum-containing medium. Cells Tissues Organs. 2011;193:344–365. doi: 10.1159/000321160. [DOI] [PubMed] [Google Scholar]
- 38.Marchionni C, Bonsi L, Alviano F, et al. Angiogenic potential of human dental pulp stromal (stem) cells. Int J Immunopathol Pharmacol. 2009;22:699–706. doi: 10.1177/039463200902200315. [DOI] [PubMed] [Google Scholar]
- 39.Janebodin K, Zeng Y, Buranaphatthana W, et al. VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. J Dent Res. 2013;92:524–531. doi: 10.1177/0022034513485599. [DOI] [PubMed] [Google Scholar]
- 40.Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Bento LW, Zhang Z, Imai A, et al. Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res. 2013;92:51–57. doi: 10.1177/0022034512466263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Gong Q, Ling J, et al. Role of miR-424 on angiogenic potential in human dental pulp cells. J Endod. 2014;40:76–82. doi: 10.1016/j.joen.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 43.Kim JJ, Kim SJ, Kim YS, et al. The role of SIRT1 on angiogenic and odontogenic potential in human dental pulp cells. J Endod. 2012;38:899–906. doi: 10.1016/j.joen.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Dissanayaka WL, Zhan X, Zhang C, et al. Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. J Endod. 2012;38:454–463. doi: 10.1016/j.joen.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima M, Iohara K. Regeneration of dental pulp by stem cells. Adv Dent Res. 2012;23:313–319. doi: 10.1177/0022034511405323. [DOI] [PubMed] [Google Scholar]
- 46.Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- 47.Derringer KA, Jaggers DC, Linden RW. Angiogenesis in human dental pulp following orthodontic tooth movement. J Dent Res. 1996;75:1761–1766. doi: 10.1177/00220345960750100901. [DOI] [PubMed] [Google Scholar]
- 48.Derringer KA, Linden RW. Enhanced angiogenesis induced by diffusible angiogenic growth factors released from human dental pulp explants of orthodontically moved teeth. Eur J Orthod. 1998;20:357–367. doi: 10.1093/ejo/20.4.357. [DOI] [PubMed] [Google Scholar]
- 49.Derringer KA, Linden RW. Angiogenic growth factors released in human dental pulp following orthodontic force. Arch Oral Biol. 2003;48:285–291. doi: 10.1016/s0003-9969(03)00008-6. [DOI] [PubMed] [Google Scholar]
- 50.Derringer KA, Linden RW. Vascular endothelial growth factor, fibroblast growth factor 2, platelet derived growth factor and transforming growth factor beta released in human dental pulp following orthodontic force. Arch Oral Biol. 2004;49:631–641. doi: 10.1016/j.archoralbio.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Grünheid T, Morbach BA, Zentner A. Pulpal cellular reactions to experimental tooth movement in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:434–441. doi: 10.1016/j.tripleo.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Derringer K, Linden R. Epidermal growth factor released in human dental pulp following orthodontic force. Eur J Orthod. 2007;29:67–71. doi: 10.1093/ejo/cjl059. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima M, Reddi AH. The application of BMPs to dental tissue engineering. Nat Biotech. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 54.Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013–1016. doi: 10.1016/s0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 55.Bagri A, Kouros-Mehr H, Leong KG, Plowman GD. Use of anti-VEGF adjuvant therapy in cancer: challenges and rationale. Trends Mol Med. 2010;16:122–132. doi: 10.1016/j.molmed.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Wang R, Chadalavada K, Wishire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 57.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 58.Gerwins P, Sköldenberg E, Claesson-Welsh L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit Rev Oncol Hematol. 2000;34:185–194. doi: 10.1016/s1040-8428(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 59.Holderfield MT, Hughes CCW. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-b in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 60.Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32A:2413–2422. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- 61.Grando Mattuella L, Westphalen Bento L, de Figueiredo JA, et al. Vascular endothelial growth factor and its relationship with the dental pulp. J Endod. 2011;37:26–30. doi: 10.1016/j.joen.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Mullane EM, Dong Z, Sedgley CM, et al. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J Dent Res. 2008;87:1144–1148. doi: 10.1177/154405910808701204. [DOI] [PubMed] [Google Scholar]
- 63.Virtej A, Løes S, Iden O, et al. Vascular endothelial growth factors signaling in normal human dental pulp: a study of gene and protein expression. Eur J Oral Sci. 2013;12:92–100. doi: 10.1111/eos.12019. [DOI] [PubMed] [Google Scholar]
- 64.Güven G, Altun C, Günhan O, et al. Co-expression of cyclooxygenase-2 and vascular endothelial growth factor in inflamed human pulp: an immunohistochemical study. J Endod. 2007;33:18–20. doi: 10.1016/j.joen.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Scheven BA, Man J, Millard JL, et al. VEGF and odontoblast like cells: stimulation by low frequency ultrasound. Arch Oral Biol. 2009;54:185–191. doi: 10.1016/j.archoralbio.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biology. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. reviews3005.1– 3005.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blaber M, DiSalvo J, Thomas KA. X-ray crystal structure of human acidic fibroblast growth factor. Biochemistry. 1996;35:2086–2094. doi: 10.1021/bi9521755. [DOI] [PubMed] [Google Scholar]
- 68.Khurana R, Simons M. Insights from angiogenesis trials using fibroblast growth factor for advanced arteriosclerotic disease. Trends Cardiovasc Med. 2003;13:116–122. doi: 10.1016/s1050-1738(02)00259-1. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi N, Hayashi Y, Murakami M, et al. Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony. Oral Dis. 2015;21:113–122. doi: 10.1111/odi.12227. [DOI] [PubMed] [Google Scholar]
- 70.Li Z, Sae-Lim V. Comparison of acidic fibroblast growth factor on collagen carrier with calcium hydroxide as pulp capping agents in monkeys. Dent Traumatol. 2007;23:278–286. doi: 10.1111/j.1600-9657.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 71.Hannink M, Donoghue DJ. Structure and function of platelet-derived growth factor (PDGF) and related proteins. Biochim Biophys Acta. 1989;989:1–10. doi: 10.1016/0304-419x(89)90031-0. [DOI] [PubMed] [Google Scholar]
- 72.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 73.Matsuoka J, Grotendorst GR. Two peptides related to platelet-derived growth factor are present in human wound fluid. Proc Natl Acad Sci U S A. 1989;86:4416–4420. doi: 10.1073/pnas.86.12.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thurston G. Role of angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–68. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 75.El Karim IA, Linden GJ, Irwin CR, Lundy FT. Neuropeptides regulate expression of angiogenic growth factors in human dental pulp fibroblasts. J Endod. 2009;35:829–833. doi: 10.1016/j.joen.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Haas TL, Milkiewicz M, Davis SJ, et al. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H1540–H1547. doi: 10.1152/ajpheart.2000.279.4.H1540. [DOI] [PubMed] [Google Scholar]
- 77.Zheng L, Amano K, Iohara K, et al. Matrix metalloproteinase-3 accelerates wound healing following dental pulp injury. Am J Pathol. 2009;175:1905–1914. doi: 10.2353/ajpath.2009.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muromachi K, Kamio N, Narita T, et al. MMP-3 provokes CTGF/CCN2 production independently of protease activity and dependently on dynamin-related endocytosis, which contributes to human dental pulp cell migration. J Cell Biochem. 2012;113:1348–1358. doi: 10.1002/jcb.24007. [DOI] [PubMed] [Google Scholar]
- 79.Ozeki N, Yamaguchi H, Kawai R, et al. Cytokines induce MMP-3-regulated proliferation of embryonic stem cell-derived odontoblast-like cells. Oral Dis. 2014;20:505–513. doi: 10.1111/odi.12165. [DOI] [PubMed] [Google Scholar]
- 80.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 81.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 82.Pan S, Dangaria S, Gopinathan G, et al. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling: potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev. 2013;9:655–667. doi: 10.1007/s12015-013-9442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 84.Limjeerajarus CN, Osathanon T, Manokawinchoke J, Pavasant P. Iloprost up-regulates vascular endothelial growth factor expression in human dental pulp cells in vitro and enhances pulpal blood flow in vivo. J Endod. 2014;40:925–930. doi: 10.1016/j.joen.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 85.Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci. 1998;19:26–37. doi: 10.1016/s0165-6147(97)01147-4. [DOI] [PubMed] [Google Scholar]
- 86.Min KS, Lee YM, Hong SO, Kim EC. Simvastatin promotes odontoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J Endod. 2010;36:447–452. doi: 10.1016/j.joen.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Kim MK, Park HJ, Kim YD, et al. Hinokitiol increases the angiogenic potential of dental pulp cells through ERK and p38MAPK activation and hypoxia-inducible factor-1α (HIF-1α) upregulation. Arch Oral Biol. 2014;59:102–110. doi: 10.1016/j.archoralbio.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 88.Trimmel K, Cvikl B, Müller HD, et al. L-mimosine increases the production of vascular endothelial growth factor in human tooth slice organ culture model. Int Endod J. 2014 doi: 10.1111/iej.12307. [DOI] [PubMed] [Google Scholar]
- 89.Kajiya M, Shiba H, Komatsuzawa H, et al. The antimicrobial peptide LL37 induces the migration of human pulp cells: a possible adjunct for regenerative endodontics. J Endod. 2010;36:1009–1013. doi: 10.1016/j.joen.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 90.Izumi T, Eida T, Matsumoto N, Inoue H. Immunohistochemical localization of metallothionein in dental pulp after cavity preparation of rat molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e133–e137. doi: 10.1016/j.tripleo.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 91.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus. FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 92.Delamaire M, Maugendre D, Moreno M, et al. Impaired leukocyte functions in diabetic patients. Diabetes Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 93.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 94.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 95.Garber SE, Shabahang S, Escher AP, Torabinejad M. The effect of hyperglycemia on pulpal healing in rats. J Endod. 2009;35:60–62. doi: 10.1016/j.joen.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 96.Maurer AM, Zhou B, Han ZC. Roles of platelet factor 4 in hematopoiesis and angiogenesis. Growth Factor. 2006;24:242–252. doi: 10.1080/08977190600988225. [DOI] [PubMed] [Google Scholar]