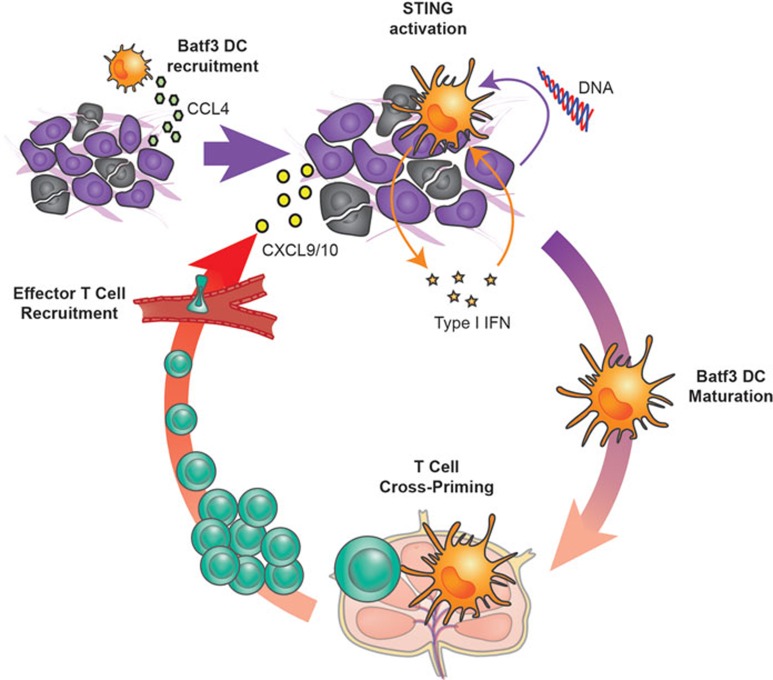
Figure 1.
A central role of Batf3 DCs and the STING pathway in orchestrating anti-tumor T cell responses. Tumor cells displaying a T cell-inflamed phenotype produce chemokines, such as CCL4, which recruit Batf3 DCs. Tumor cell turnover and perhaps death results in the liberation of DNA and other DAMPs that gain access to infiltrating APCs, including DCs. Cytosolic DNA in APCs activates the STING pathway, resulting in the secretion of type I IFNs and also of the chemokines CXCL9 and CXCL10. Tumor endothelial cells can also contribute to the production of type I IFNs. This cytokine subsequently facilitates the activation of tumor-infiltrating Batf3 DCs, which take up tumor-associated antigens and migrate via the lymphatics to the tumor-draining lymph node, where they cross-prime tumor-specific CD8+ T cells. The activated CD8+ T cells undergo clonal expansion in the tumor-draining lymph node and traffic via the bloodstream. They are recruited back to the tumor microenvironment under the influence of CXCL9 and CXCL10 also released by Batf3 DCs in the tumor microenvironment. Dynamic movement of infiltrated CD8+ effector T cells results in direct contact with tumor cells, which can lead to tumor cell death. If tumor cells are not completely eliminated, then immune inhibitory pathways suppress T cell activation as part of a negative feedback loop.