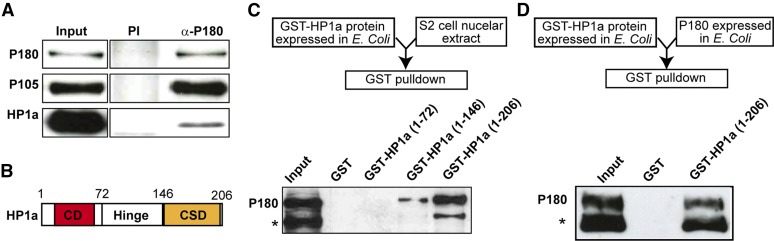
Figure 1.
P180 and HP1a interact directly. (A) IP of P180 in nuclear extracts from cultured Schneider S2 cells. The large (P180) and medium (P105) subunits of CAF-1, as well as the Drosophila HP1a protein, were detected by western blot on nuclear extracts (Input), after IP with a preimmune serum (PI) or with an anti-P180 antibody (α-P180). (B) Representation of the HP1a protein, carrying a chromodomain (CD, red) in the N-terminal and a chromo shadow domain (CSD, orange) in the C-terminal. The positions of the first and last HP1a amino acids contained in the GST-fusion proteins used hereafter are indicated. (C) GST pull-down assay in nuclear extracts from cultured Schneider S2 cells, using different forms of HP1a fused to GST in N-terminal. Bottom panel: western blot anti-P180 on the nuclear extract (Input) and after the pull-down assay using the different fusion proteins. (D) GST pull-down assay using recombinant proteins expressed in E. coli. The presence of P180 in the bound fraction was analyzed by western blotting against a poly-histidine motif fused to its C-terminal. * designates a putative degradation product of the P180 protein.