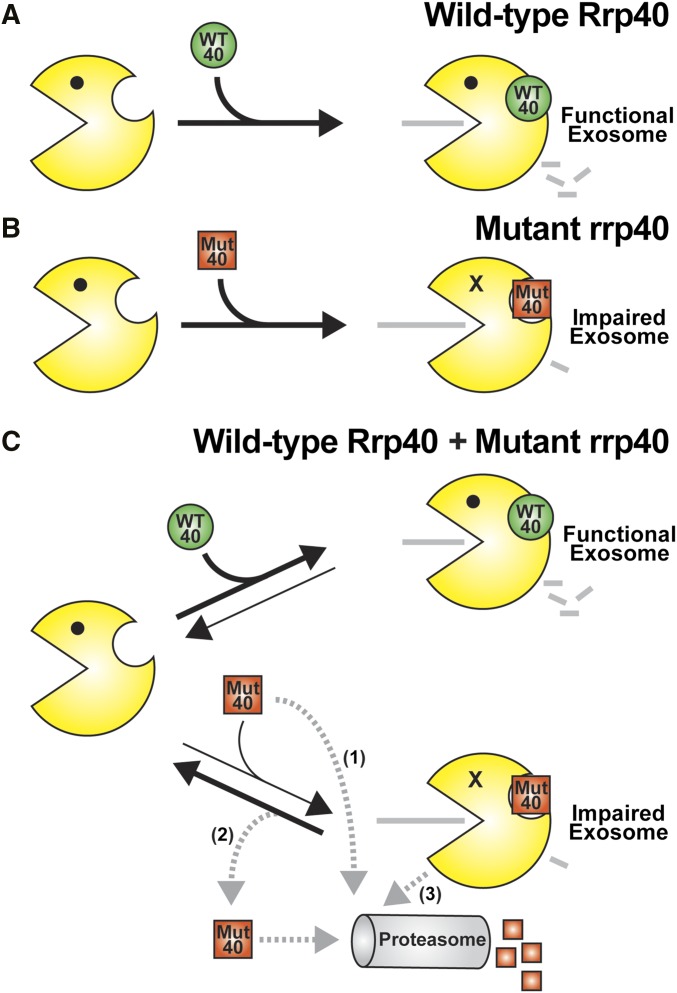
Figure 9.
Model for exosome assembly and function. (A) When cells express wild-type Rrp40 (WT 40) as the only copy of Rrp40, the exosome assembles properly to produce a fully functional exosome. (B) When cells express variant rrp40-W195R (Mut 40) as the only copy of Rrp40, the exosome shows impaired function as evidenced by a modest decrease in cell growth, altered levels of exosome target transcripts, and subunit instability. (C) When cells express both wild-type Rrp40 (WT 40) and variant rrp40-W195R (Mut 40), the rrp40-W195R is highly unstable and degraded rapidly in a proteasome-dependent manner. As indicated by the black arrows depicting RNA exosome complex assembly and disassembly, the rrp40-W195R protein (Mut 40) could be assembled into the RNA exosome less efficiently than the wild-type Rrp40 (WT 40), or the exosome complex assembled with rrp40-W195R (Mut 40) could be disassembled more rapidly than the exosome containing wild-type Rrp40. Importantly, reduced association of rrp40-W195R with the exosome in cells expressing wild-type Rrp40 is supported by native gel and glycerol gradient analysis. Several possible routes for degradation of rrp40-W195R (Mut 40) exist (gray dashed arrows): (1) Mut 40 subunit could be degraded directly without ever being incorporated into the RNA exosome; (2) Mut 40 subunit that results from disassembly of the exosome complex could be targeted for degradation; or (3) the entire RNA exosome assembled with Mut 40 could be targeted for degradation. Further studies will be required to distinguish between these possible mechanisms for rapid, proteasome-mediated turnover of the rrp40 variant.