Table 2.
Evidence table for TTD versus PC alone in terminally ill adults GRADE evidence profile
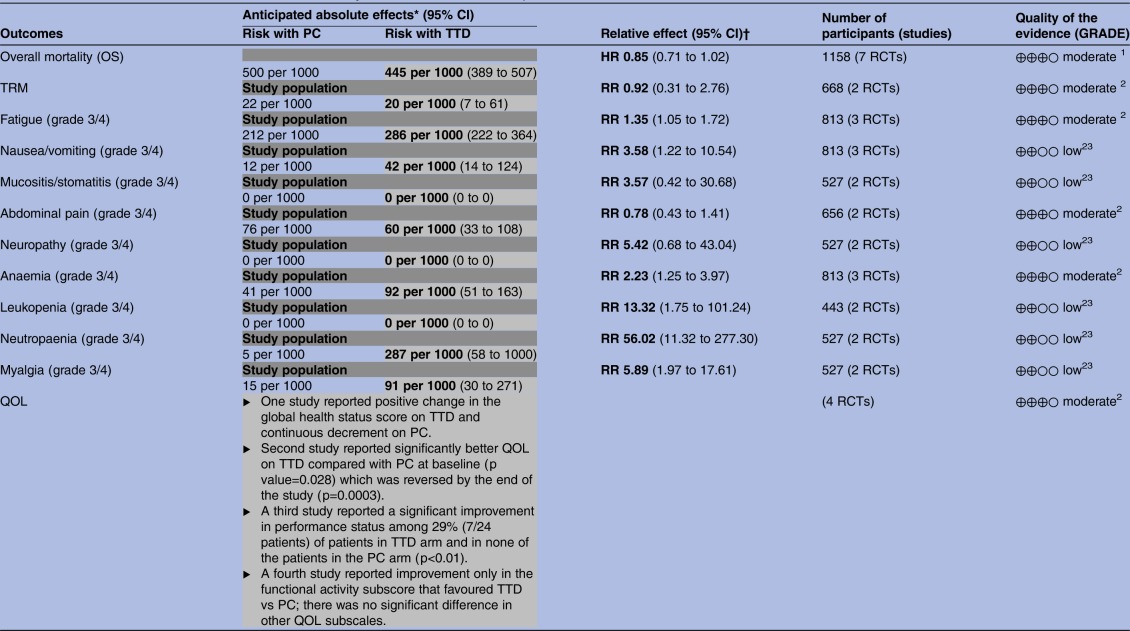 |
GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
1. High heterogeneity between studies.
2. Majority of studies not reporting data on outcome.
3. Wide CIs.
Any text which is either bold or in grey shading represents the risk associated with TTD.
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†<1: Poorer results with PC; >1: poorer results with TTD; 1=no difference between effects of PC and TTD.
GRADE, Grading of Recommendations Assessment, Development and Evaluation; OS, overall survival; PC, palliative care; QOL, quality of life; RCTs, randomised controlled trials; RR, risk ratio; TRM, treatment-related mortality; TTD, treatment targeted at underlying disease.
