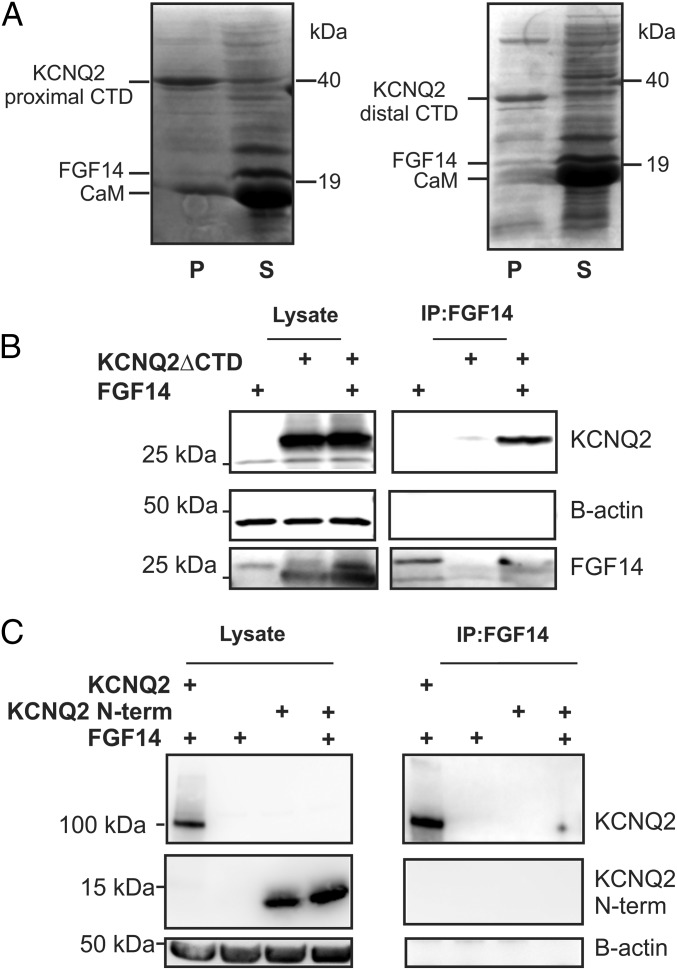
Fig. 6.
FGF14 binding to KCNQ2 does not require the C terminus nor is the KCNQ2 N terminus sufficient for binding. (A) Copurification experiments with a 6xHis-tagged KCNQ2 C terminus show that calmodulin (CaM) binds, whereas FGF14 does not. A different KCNQ2 C terminus construct, without the CaM binding site, copurifies neither FGF14 nor CaM. (B) Immunoprecipitation of FGF14 is still capable of immunoprecipitating a KCNQ2 construct that lacks a C terminus. Omission of either protein does not yield a significant signal. (C) Immunoprecipitation of FGF14 does not coimmunoprecipitate HA-tagged KCNQ2 N terminus. Coimmunoprecipitation of the full-length HA-tagged KCNQ2 is shown as a positive control.