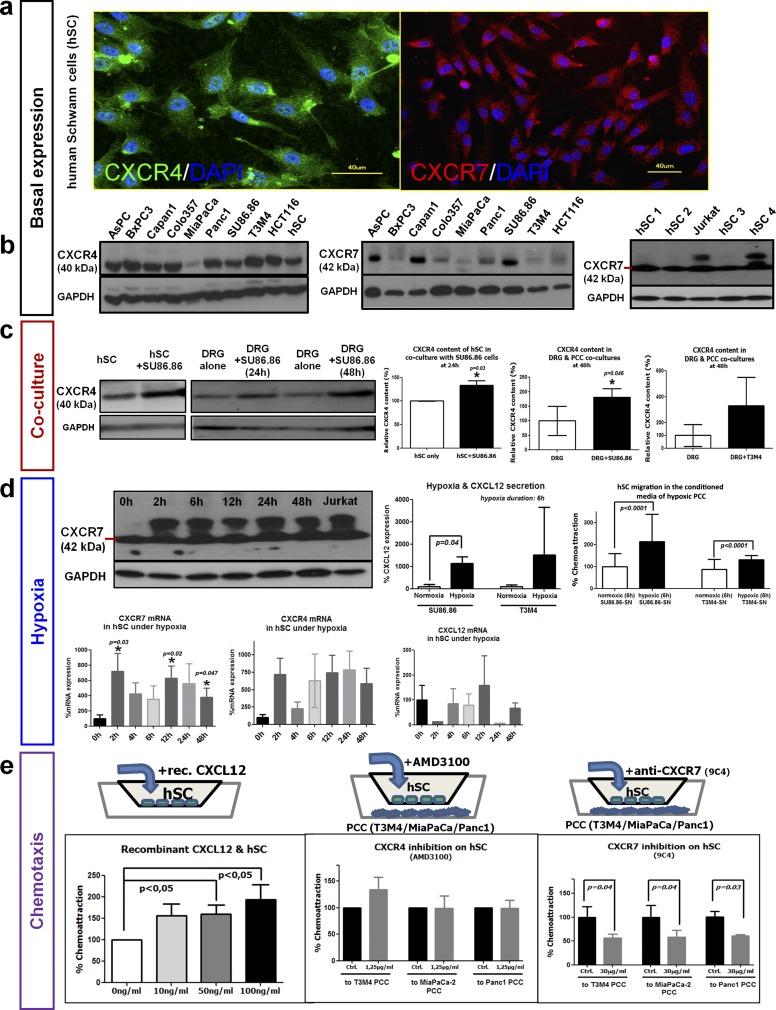
Fig. S2.
Basal and regulated expression of CXCR4 and CXCR7 in SC. (A and B) Primary hSC were found to express CXCR4 and CXCR7 via immunofluorescence (A) or via immunoblotting (B) in simultaneous comparison with human PCC lines and one human colon cancer cell line (HCT116). (C, Left) hSC were cocultured with the SU86.86 PCC line, and at 24 h their CXCR4 content was compared with that of monocultured hSC via immunoblotting. (Right) Newborn rat DRG that contain SC were also cocultured with SU86.86 or T3M4 PCC and at 48 h of coculture exhibited higher CXCR4 content than monocultured DRG (unpaired t test). (D, Upper Left) hSC that were exposed to increasing periods of hypoxia (0–48 h) exhibited both the 42-kDa native CXCR7 isoform and another larger isoform that also was present in Jurkat T cells. (Lower Left) At the mRNA level, 2- or 12-h hypoxia exposure led to significant up-regulation of CXCR7 in hSC, with a similar tendency for CXCR4. However, CXCL12 expression in hSC was not influenced by hypoxia. (Right) Hypoxia exposure enhanced CXCL12 expression in SU86.86 and T3M4 PCC lines. Accordingly, the addition of the supernatant of hypoxia-treated (6 h) PCC lines to the medium of hSC enhanced the hSC transmigration in Boyden chambers (Mann–Whitney u test). (E, Left) hSC were placed in the upper chamber of a Boyden Transwell chamber and were supplied with increasing doses of recombinant human CXCL12. The number of transmigrating hSC was determined and expressed as percent of control. (Center and Right) Similarly, T3M4 or MiaPaCa-2 human PCC were placed in the lower chamber, and hSC in the upper compartment were pretreated with the CXCR4 inhibitor AMD3100 or with a CXCR7-blocking antibody (9C4). The solvent for AMD3100 or a nonimmunized mouse IgG1 antibody was used as control (unpaired t test). Experiments were repeated three times.