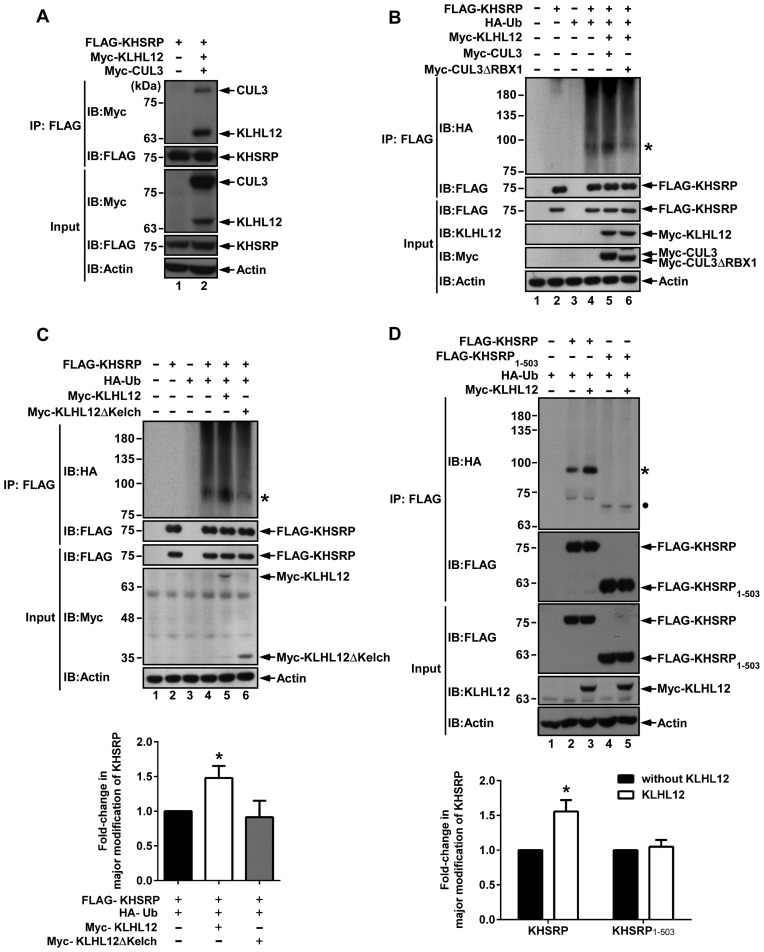
Figure 3.
KHSRP can be ubiquitinated by the KLHL12-based CUL3 complex. (A) KHSRP was found to form a complex with KLHL12 and CUL3. FLAG-KHSRP, Myc-KLHL12 and Myc-CUL3 expression vectors were co-transfected to 293T cells. The cell lysates were immunoprecipitated with Anti-FLAG M2 affinity gel, and then analyzed by western blot using anti-Myc and anti-FLAG antibodies. (B) 293T cells were co-transfected with FLAG-KHSRP, HA-Ub, Myc-KLHL12, Myc-CUL3 or Myc-CUL3ΔRBX1, and then treated with 20 μM MG132 for 4 h before harvesting. Cell lysates were immunoprecipitated with Anti-FLAG M2 affinity gel and resolved by SDS-PAGE, and KHSRP ubiquitination was detected by anti-HA antibody. The asterisk (*) represents the major modification of KHSRP. KHSRP, KLHL12, CUL3 and CUL3ΔRBX1 were visualized by individual antibodies against FLAG, KLHL12 and Myc. (C) Various constructs expressing KHSRP, Ub, KLHL12 or KLHL12 truncated form (KLHL12ΔKelch) were co-transfected to 293T cells. After 48 h, cells were treated with 20 μM MG132 for 4 h before harvesting, and lysates were then subjected to immunoprecipitation using Anti-FLAG M2 affinity gel. KHSRP ubiqitination was assessed by western blot, and KHSRP, KLHL12 and KLHL12ΔKelch were visualized using anti-FLAG and anti-Myc antibodies. (D) 293T cells were co-transfected with various indicated constructs and subsequently treated with 20 μM MG132 for 4 h before harvesting. Western blots were utilized to analyze ubiquitinated proteins. The asterisk (*) represents the major modification of KHSRP. The circle represents the major modification of KHSRP1-503. For both (C) and (D), levels of KHSRP or KHSRP1–503 with major modification were respectively normalized against levels of FLAG-KHSRP or FLAG-KHSRP1–503, and fold-changes over control reactions without KLHL12 were calculated and plotted.