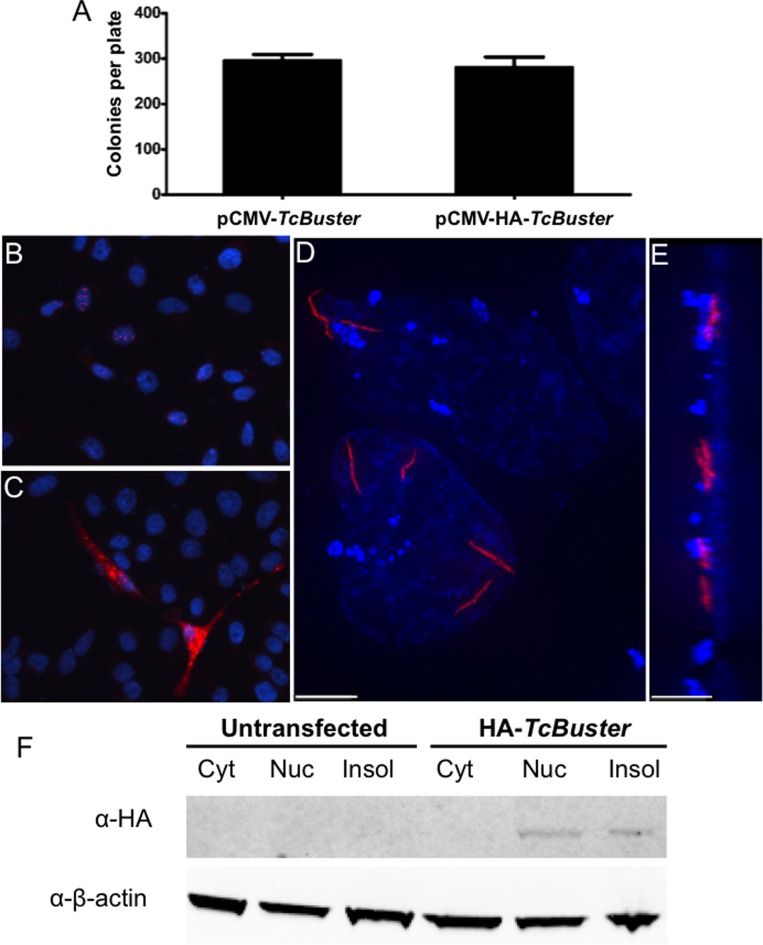
Figure 1.
TcBuster transposase is localized to rodlet structures in human tissue culture cells. (A) To test the activity of the HA-tagged TcBuster transposase, HEK-293 cells were transfected with the transposase plasmid indicated and the pTcBNeo neomycin-resistance transposon plasmid at a 1:5 ratio, n = 3 per group. After 48 h, the cells were split into selection media and colonies were counted after two weeks of selection. To localize the transposase protein, HeLa cells were transfected with 1 μg of pCMV-HA-TcBuster plasmid. The following day, the cells were fixed with formaldehyde and stained to detect the HA tag (red) and DNA with DAPI (blue). Representative image shows nuclear rodlet inclusions (B), non-representative image showing extreme overexpression phenotype with cytoplasmic inclusions that were observed rarely (C). No red staining could be found at this exposure on IgG only or untagged controls (not shown). Imaging of HA-TcBuster was performed on a deconvolution microscope (D). In (E), the image shown in (D) has been rotated 90° such that the left is the top of the cells and the right is the bottom of the cells. The scale bar represents 5 μm in (D) and (E). (F) Cytoplasmic (Cyt), nuclear (Nuc), and insoluble (Insol) protein lysates prepared from transfected HEK-293 cells were subjected to immunoblotting for the HA-tagged transposase (top row) and the β-actin loading control (bottom row). The transposase was detected at the expected size in the nuclear and insoluble fractions.