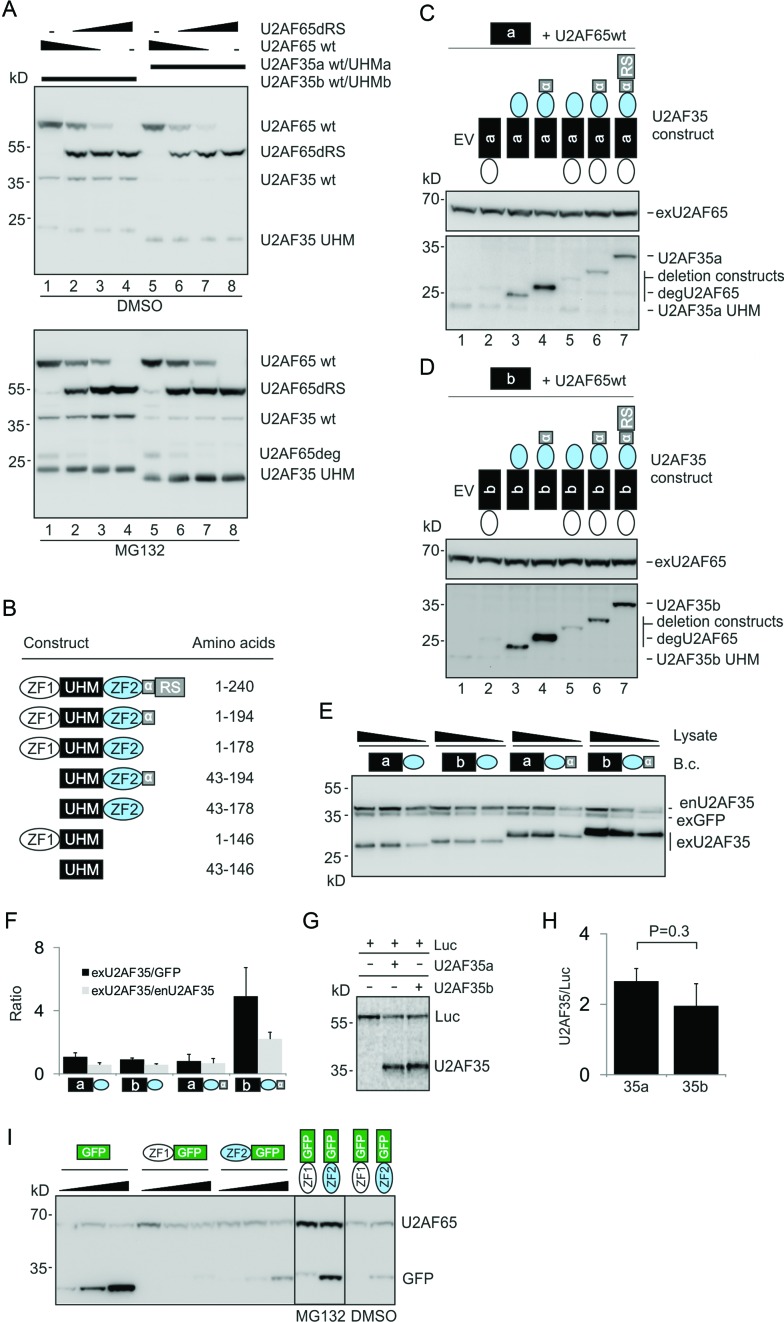
Figure 7.
The role of U2AF35 domains in isoform-dependent expression. (A) UHM domains alone are not sufficient for differential expression of full-length U2AF35 isoforms. HEK293 cells were transfected with constant amounts of 1:1 mixtures of the wild-type and UHM U2AF35a plasmids or the wild-type and UHM U2AF35b plasmids. The plasmids were supplemented with varying ratios of U2AF65/U2AF65dRS plasmids (150, 50, 15 and 0/0, 100, 135 and 150 ng). The U2AF65ΔRS plasmid was used as a transfection control and to formally exclude that the U2AF65 RS domain can affect the isoform-specific expression through additional contacts with U2AF35. MG132 or DMSO was added 36 hrs later. Immunoblots were incubated with the Xpress antibody, which detects the N-terminal part (∼25 kD) of U2AF65deg (degradation product of U2AF65). U2AF35a was visible only in MG132-treated cells. (B) Summary of U2AF35 deletion constructs. Their alignments are in Supplementary Figure S17. The α6 helix (residues 179–194) is denoted by α. (C and D) The importance of U2AF35 domains for expression of U2AF35a (C) and U2AF35b (D). The deletion plasmids or empty vectors (EV) were cotransfected with constant amounts of wild-type U2AF65 and UHM-only U2AF35 constructs (150 ng/ml each); membranes were incubated with the Xpress antibody. Plasmid symbols correspond to those in panel (B). (E) The α6 helix of U2AF35 is necessary for differential expression of U2AF35a and U2AF35b. Concentration of the indicated bicistronic vectors (B.c.) was 150 ng/ml. Cell lysates (40, 20 and 10 μg for constructs lacking α6 and 30, 15 and 7.5 μg for constructs containing α6) were incubated with U2AF35 and His antibodies. (F) Signal intensities measured for panel (E). Error bars indicate SDs. (G) Cell-free translation of wild-type U2AF35a and U2AF35b constructs. Luc-expressing plasmid was used as a control. (H) Signal intensities for panel (G). Error bars indicate SDs for two independently cloned and sequence-verified plasmids separately translated in vitro. (I) U2AF35 ZF domains reduce expression of the C-terminal GFP. Expression of the ZF1-GFP constructs could be visualized only after incubation with MG132 (right panel). Blots were incubated with the Xpress antibody.