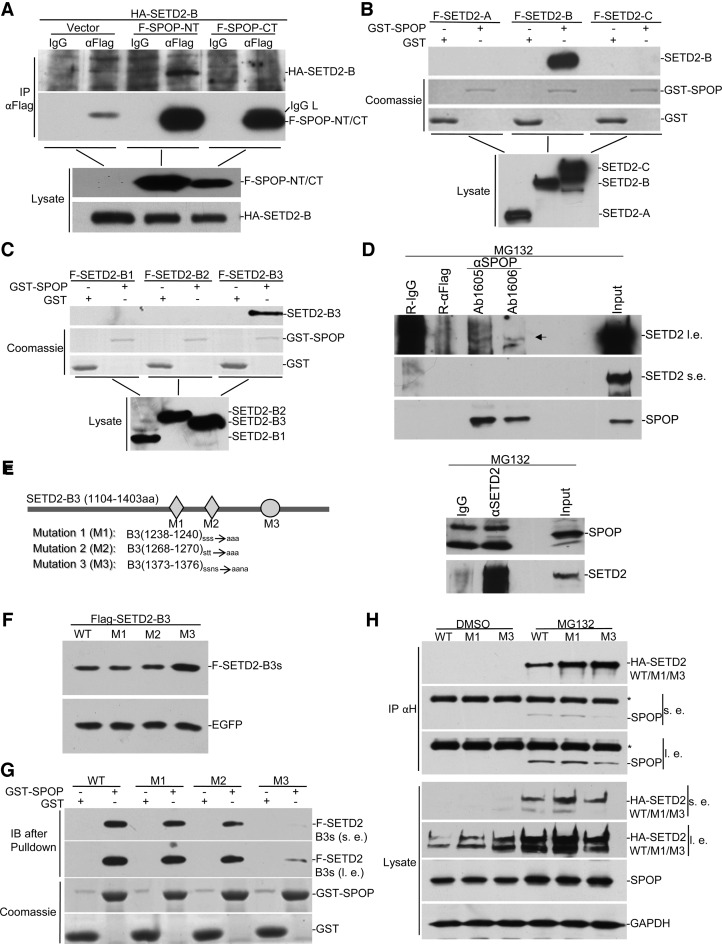
Figure 2.
SPOP interacts with SETD2. (A) HA-SETD2-B. was transfected into HEK293 with FLAG-SPOP-NT or FLAG-SPOP-CT. Lysates were immunoprecipitated with anti-FLAG, followed by anti-HA Western blot. (B and C) SETD2 domains were mapped for SPOP binding by GST pulldown. GST-SPOP was expressed and purified from bacteria. FLAG-tagged different fragments of SETD2 were expressed in HEK293 and lysates were subject to GST pulldown assays. (D) Immunoprecipitation of endogenous SETD2 and SPOP. HEK293 cell were treated with MG132 and co-immunoprecipitation was carried out with SETD2 antibody and two different SPOP antibodies, as indicated. (E) Schematic representation of mutations of predicted SPOP binding sites. (F) HEK293 cells were transfected with equal amounts of EGFP and FLAG-tagged SETD2-B3 mutants. Lysates were assayed by Western blot. (G) The wild type and mutants of SETD2-B3 fragment were subjected to GST-pulldown as in B and C. SPOP pulled down less M3 compared with wild type and other mutants. (H) The wild type and the M1 and M3 versions of full-length SETD2 were respectively expressed in HEK293 cells together with FLAG-SPOP. *indicates IgG heavy chain, s.e. for short exposure, and l. e. for long exposure.