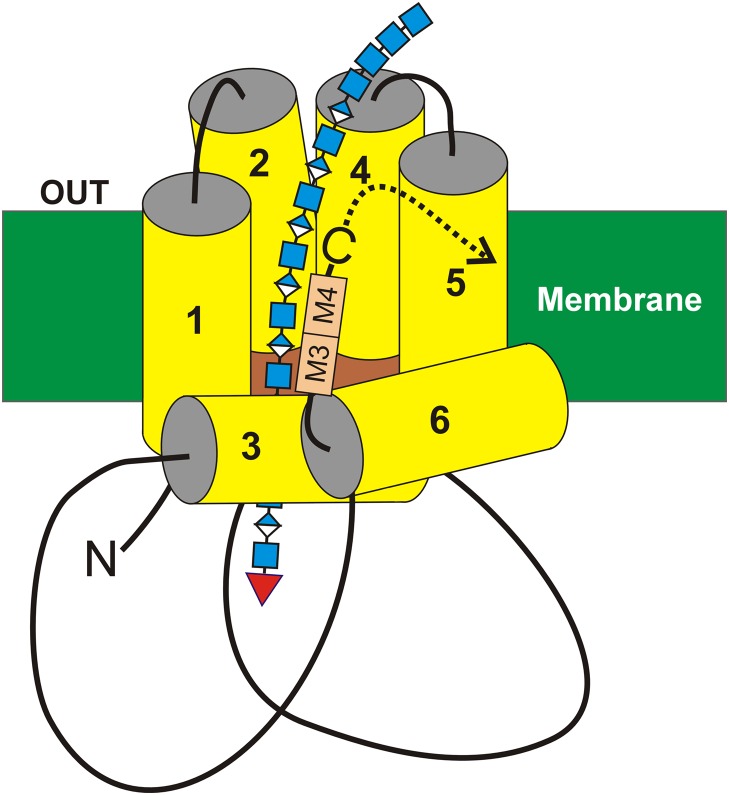
Fig. 7.
A model for interaction of the HAS tandem-motif region with HA. The organization (Heldermon et al. 2001a; Kumari and Weigel 2005; Kumari et al. 2006) of the four transmembrane (MD1 & MD2 and MD4 & MD5) and two (MD3 and MD6) amphipathic MDs of SeHAS (yellow) are shown with the growing HA-UDP chain, containing alternating GlcNAcβ1,4 (blue squares) and GlcUAβ1,3 (blue-white diamonds) attached to UDP (red inverted triangle) at the reducing end. Preliminary results indicate that the nonreducing end contains a chitin oligomer cap with four GlcNAc residues, which is the primer on which HA synthesis is initiated (Weigel 2015; Weigel et al. 2015). The relative positions of the tandem B-X7-B motifs (A), M3 and M4, are indicated (tan boxes). In streptococcal HAS enzymes the C-terminus (-C) is just after the tandem-motif, whereas in vertebrate Class I HAS enzymes the additional ~140 amino acid sequence continues after the tandem-motif (dotted curved arrow). This figure is available in black and white in print and in color at Glycobiology online.