Abstract
The human prostate is a gland of the male reproductive tract, which together with the seminal vesicles, is responsible for most seminal fluid production. It is a common site of cancer, and unlike other glands, it typically enlarges in aging men. In flies, the male accessory glands make many major seminal fluid components. Like their human equivalents, they secrete proteins from several conserved families, including proteases, lectins, and cysteine-rich secretory proteins, some of which interact with sperm and affect fertility. A key protein, sex peptide, is not conserved in vertebrates but plays a central role in mediating long-term effects on females after mating. Although postmitotic, one epithelial cell type in the accessory glands, the secondary cell, continues to grow in adults. It secretes microvesicles called exosomes from the endosomal multivesicular body, which, after mating, fuse with sperm. They also appear to affect female postmating behavior. Remarkably, the human prostate epithelium also secretes exosomes, which fuse to sperm in vitro to modulate their activity. Exosomes from prostate and other cancer cells are increasingly proposed to play fundamental roles in modulating the tumor microenvironment and in metastasis. Here we review a diverse accessory gland literature, which highlights functional analogies between the male reproductive glands of flies and humans, and a critical role for extracellular vesicles in allowing seminal fluid to promote male interests within the female. We postulate that secondary cells and prostate epithelial cells use common mechanisms to control growth, secretion, and signaling, which are relevant to prostate and other cancers, and can be genetically dissected in the uniquely tractable fly model.
1. Introduction
As discussed in the chapter “Modeling Human Cancers in Drosophila” by Sonoshita and Cagan in this volume, Drosophila melanogaster has provided a powerful system to model many different aspects of cancer biology. These include features described as hallmarks of cancer by Hanahan and Weinberg (2000, 2011), such as dysregulation of cell growth and proliferation, evasion from apoptosis, and invasive and metastatic properties (also reviewed in Rudrapatna, Cagan, & Das, 2012; Tipping & Perrimon, 2014). Genetic approaches in flies led to the identification and characterization of many of the intercellular signaling cascades that drive tumorigenesis (Perrimon, Pitsouli, & Shilo, 2012). In some cases, such as the control of epithelial polarity, they highlighted a new cell biological process that is part of the cancer regulatory network (Grifoni, Froldi, & Pession, 2013). Flies have also started to be employed in the study of tumor microenvironment and tumor–stroma interactions (Patel & Edgar, 2014). Furthermore, new candidate oncogenes and tumor suppressors, which are increasingly identified in genome sequence analysis of patient tumors (Martincorena & Campbell, 2015; Mitchell & Neal, 2015), can be tested in Drosophila.
Most cancer-related studies in flies have focused on developing tissues, particularly the larval imaginal discs and brain, because cells in these structures are still dividing (Tipping & Perrimon, 2014), unlike many adult cell types. However, proliferating follicular epithelial cells of the adult ovary have been particularly useful in analyzing the cancer-relevant mechanisms linking cell growth, signaling, polarity, and migration (Klusza & Deng, 2011; Rosales-Nieves & González-Reyes, 2014). Furthermore, in the last 10 years, numerous studies of stem cell regulation in the Drosophila midgut have not only revealed parallels with human intestinal stem cells (Nászai et al., 2015) but also suggested new control mechanisms that may be relevant to colorectal cancer (Panayidou & Apidianakis, 2013). These adult systems provide tissue- and cell type-specific insights into cancer mechanisms that complement data emerging from other fly tumor models (Bell & Thompson, 2014).
1.1. Modeling Adenocarcinoma
The majority of common human cancers are formed from glandular tissue, so-called adenocarcinomas, including most pancreatic (Ryan, Hong, & Bardeesy, 2014), lung (Ding et al., 2008), breast (Li & Daling, 2007), esophageal (Edgren, Adami, Weiderpass, & Nyrén, 2013), stomach (Shah et al., 2011), intestinal (Weitz et al., 2005), and prostate (Humphrey, 2012) tumors. Analysis of secretion from Drosophila glands and secretory epithelia has been undertaken in tissues like the Malpighian tubules (Cabrero et al., 2014) and larval salivary gland, where some detailed cell biological studies have been performed (Burgess et al., 2012, 2011; Torres, Rosa-Ferreira, & Munro, 2014). But generally, even analysis of genes that selectively promote tumorigenesis in glands, like the conserved receptor tyrosine kinase Ret, which is mutated in multiple endocrine neoplasia 2 (MEN2), has to date been undertaken in nonglandular fly tissue (Das & Cagan, 2013).
As discussed later, research into the human prostate and prostate cancer has lagged behind several other cancer types, particularly because of current limitations with mouse models. Recently, studies of the accessory gland in male flies have revealed surprising parallels with the human prostate epithelium (Corrigan et al., 2014; Ito et al., 2014; Leiblich et al., 2012; Xue & Noll, 2002), which indicate that this gland can be employed to model some prostate-specific cellular mechanisms, as well as processes potentially associated with other male reproductive glands like the seminal vesicles (see Sections 5–7). In addition, the remarkable subcellular structure of one epithelial cell type in this gland, known as the secondary cell, has opened up new opportunities to study secretory mechanisms, particularly those involving secretion of microvesicles (Corrigan et al., 2014). These mechanisms have not been extensively studied in any in vivo model to date but are likely to be relevant to all cancers and potentially other diseases affecting secretory functions.
2. The Prostate
2.1. The Human Prostate
The prostate is a multilobular exocrine gland, which together with the other major male accessory glands, the seminal vesicles, produces most of the seminal fluid volume (Fig. 1A; Declan, Cahill, Chandra, & Davies, 2009). The human prostate epithelium appears pseudostratified and consists of two basic cell types, basal cells and more columnar luminal secretory cells (Long, Morrissey, Fitzpatrick, & Watson, 2005). The epithelium is arranged in branched tubules connected by ducts, which also have some secretory activity. The glandular tissue is surrounded by a fibromuscular stroma. The luminal content of the tubules is pumped into the urethra early during ejaculation and mixes with sperm. Prostate secretions play an important role in sperm capacitation, which is required to induce full sperm motility and fertilization capacity (Fraser, 1997; Miah, Salma, Hamano, & Schellander, 2015). A key role for the seminal vesicles, which produce about 70% of seminal fluid volume, is to provide fructose as an energy source to power sperm motility (Aumüller & Riva, 1992). However, the latter glands also appear to affect the signaling from the female reproductive tract to the preimplantation embryo (Bromfield et al., 2014), suggesting a complex interplay between male reproductive glands and the female reproductive system postmating.
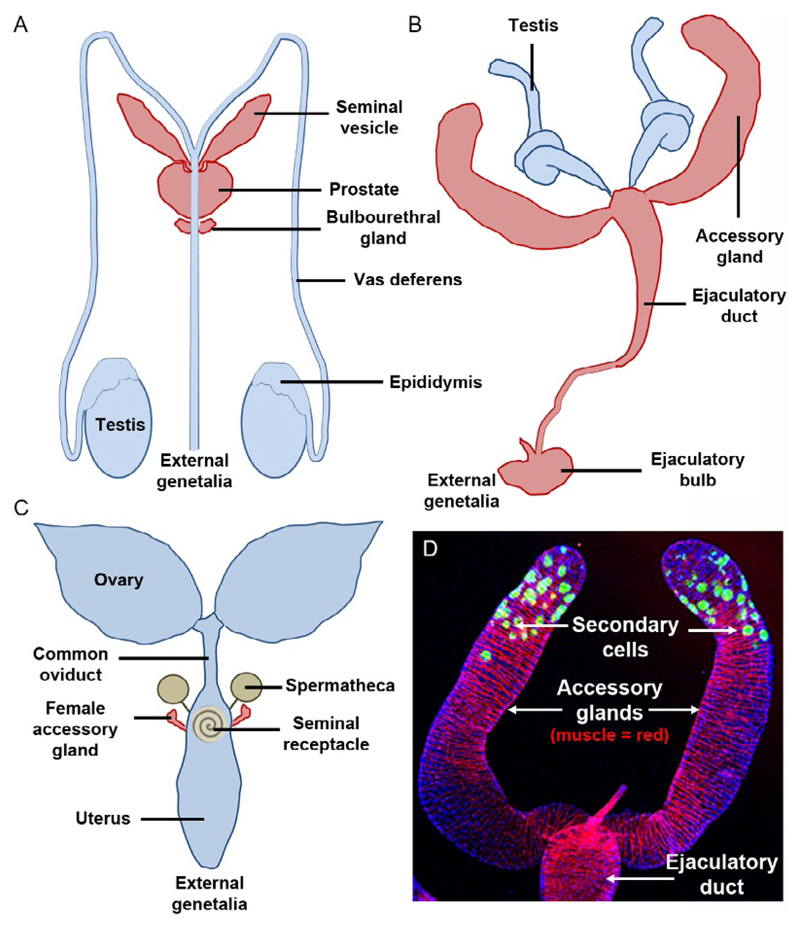
Fig. 1.
Human and fly reproductive systems. (A and B) Schematics illustrate male reproductive systems of humans (A) and flies (B). Note red-shaded secretory organs in both organisms contribute much of the volume of seminal fluid, many key proteins, and nutrients, and are critical for normal fertility. (C) Fly female reproductive system includes two different sperm storage organs (shaded green), the paired spermathecae and the seminal receptacle. They gradually release sperm after mating under the control of SP, which is itself slowly released from its binding sites on sperm. Females also have two small secretory accessory glands. (D) Confocal image of paired accessory glands (arrows). In this whole mount, the 40 secondary cells in the epithelium express GFP (green), but the more abundant main cells do not. The preparation is stained with TRITC-phalloidin, which highlights the striated muscle layer (red) surrounding the gland that undergoes peristaltic contraction upon mating, and DAPI (blue), which marks the nuclei.
Both normal prostate growth and secretion are dependent on androgens (Hayward & Cunha, 2000), linking prostate activity to the function of the testis, the major source of androgens in males. Prostate secretions include proteases, particularly those in the kallikrein class, most notably prostate-specific antigen (PSA), which has historically been employed as a biomarker for prostate cancer (Veveris-Lowe, Kruger, Walsh, Gardiner, & Clements, 2007). As discussed later, these enzymes are thought to cleave a number of substrates within the ejaculate, leading, for example, to the liquefaction of the seminal coagulum.
The prostate epithelium is also a source of secreted microvesicles called exosomes. These vesicles are formed inside multivesicular bodies (MVBs; Colombo, Raposo, & Théry, 2014), which are generally thought to be of late endosomal origin. Although one potential fate is for them to be degraded when the MVB fuses with the lysosome, they can also be released via MVB fusion to the plasma membrane. Human prostate exosomes, or prostasomes, are postulated to affect sperm activity and protect sperm from the female immune system (Aalberts, Stout, & Stoorvogel, 2013; Park et al., 2011; Ronquist, 2015). Since exosomes are increasingly implicated in cancer biology (Yu, Cao, Shen, & Feng, 2015), developing a better understanding of their normal physiological roles and regulation is becoming an important priority in modern cancer research.
2.2. Human Prostate Cancer
Although the prostate epithelium appears to have a relatively low turnover rate, the majority of men over 50 suffer from benign prostatic hyperplasia. The prostate becomes progressively enlarged, potentially leading to lower urinary tract symptoms, affecting urinary retention and frequency (Berry, Coffey, Walsh, & Ewing, 1984). A separate pathology that arises in aging men is adenocarcinoma of the prostate, which is one of the most common cancers affecting older men in the developed world (Siegel, Ma, Zou, & Jemal, 2014). Indeed, it is the second most common cause of male cancer deaths in the United Kingdom (Cancer Research UK, 2012). Many newly diagnosed cases are and will remain indolent, but when more aggressive disease progresses to its metastatic stages, drug treatments that block androgen signaling prove highly effective in most patients in the short term. However, androgen-independent tumor cells and castration-resistant prostate cancer inevitably emerge and ultimately lead to death (Schrijvers, 2007). Distinguishing the 2% of patients who are likely to advance to metastatic disease from those with indolent disease is a major clinical challenge (Attard et al., 2016; Jaiswal, Sarmad, Arora, Dasaraju, & Sarmad, 2015).
Surprisingly, androgen-independent cancer cells typically express the androgen receptor (AR) at high levels and require the receptor for growth, even though androgens themselves are not needed (Lamb, Massie, & Neal, 2014). How can the receptor continue to signal in the absence of its ligand? There are several possible explanations: AR gene amplification, which increases AR protein levels (Visakorpi et al., 1995); AR point mutations (which are much more frequent in metastatic tumors; Watson et al., 2010; Zhang et al., 2011) that can make the AR constitutively active or lead to altered steroid sensitivity (Duff & McEwan, 2005); androgen production by alternative mechanisms that circumvent drug inhibition (Titus, Schell, Lih, Tomer, & Mohler, 2005); and the influence of other signaling cascades on hormone-independent AR activity (Sharma et al., 2010; Vinall et al., 2011). Gene fusions between the AR-dependent gene TMPRSS2 (transmembrane protease serine 2) and ETS (E26 transformation-specific) genes, particularly ERG (ETS-related gene), are also implicated downstream of AR, but their role is controversial (Clark & Cooper, 2009). Key problems in current prostate cancer research are that prostate cancer cell lines have diverse androgen-dependent and -independent phenotypes, and normal AR-regulated prostate biology is not well understood, making it difficult to determine the initiating mechanisms by which androgen-independence emerges.
2.3. Mouse Models of Prostate Biology and Cancer
The mouse prostate is extremely small, and its lobular structure and epithelial morphology are quite different from the human prostate. It can therefore be difficult to relate tumor phenotypes in this organ to human cancers (Ittmann et al., 2013). Some prostate cancer models have been developed (Wu, Gong, Roy-Burman, Lee, & Culig, 2013), by expression of oncogenes like the SV40 large T antigen under androgen regulation in prostate epithelium (the transgenic adenocarcinoma of the mouse prostate model; Gingrich et al., 1997) or by prostate-specific loss of key tumor suppressor genes like Pten (Wang et al., 2003). However, the relevance of these models to human prostate cancer is still not fully established.
Orthotopic, intraosseous, and intracardiac inoculations of human cancer cells as xenografts are well established in mouse (van Weerden & Romijn, 2000), but these types of study have limitations. Not only are these experiments performed in an immunosuppressed host, but they often employ a single cancer cell line with a unique combination of genetic defects.
Cancer studies are frequently informed by the developmental biology of the cells and organs involved. Prostate epithelium-specific, Cre-induced systems for generating mutant cells at early developmental stages are available (Stanfel et al., 2006), and roles for transcription factors SOX9 and Pax2, and for FGF10, Notch, BMP, Wnt, and Hedgehog signaling (Leong & Gao, 2008; Omori et al., 2014; Peng & Joyner, 2015; Powers & Marker, 2013; Schwertfeger, 2009; Simons et al., 2012; Thomsen, Francis, & Swain, 2008; Xu, Hariharan, Rakshit, Dressler, & Wellik, 2012) have all been highlighted. However, an integrated picture of prostate development remains elusive. Even in adult epithelial tissue, the relationship between basal and luminal epithelial cells is not fully characterized (Long et al., 2005). Establishment of other in vivo systems that might be able to model at least some of the developmental, cellular, or even subcellular processes associated with the prostate epithelium could therefore provide valuable new insights into the human prostate in health and disease.
3. The Drosophila Accessory Gland—A Key Gland in the Male Reproductive System
The male reproductive system in flies, like mammals, also contains a number of secretory structures that contribute to seminal fluid. Most prominent among these are the paired accessory glands that secrete most of the total seminal fluid volume. However, other epithelial cells in the ejaculatory duct and ejaculatory bulb also contribute (Fig. 1B). Each accessory gland is lined by a simple monolayer epithelium containing two cell types with distinct gene expression patterns (Bertram, Akerkar, Ard, Gonzalez, & Wolfner, 1992), about 1000 squamous main cells and roughly 40 more cuboidal secondary cells (Bairati, 1968; Fig. 1D). The epithelium is surrounded by a thin layer of striated muscle (Susic-Jung et al., 2012), which contracts during mating under neural control (Tayler, Pacheco, Hergarden, Murthy, & Anderson, 2012), ejecting the luminal contents of the gland through the ejaculatory duct and into the female’s uterus after the transfer of sperm (Bertram, Neubaum, & Wolfner, 1996; Gilchrist & Partridge, 2000).
Male flies induce multiple behavioral changes in females to which they mate (Kubli, 2003; Sirot et al., 2009). Egg-laying rate is dramatically increased for many days, subsequent attempted matings by other males are rejected, the female’s immune response, diet, and endocrine system are modulated, and ultimately lifespan is reduced. Mutations inhibiting accessory gland development or targeted ablation of main cells by expression of diphtheria toxin strongly suppress the effects on egg-laying and remating behavior (Kalb, DiBenedetto, & Wolfner, 1993; Xue & Noll, 2000), highlighting the critical function of the accessory gland in reproductive function.
As discussed later, it is only recently that the cell biology of the adult accessory gland epithelium has attracted increased attention. However, its functions in reproductive physiology have been studied for many years (Kubli, 2003; Sirot et al., 2009), revealing central roles in fertility and in providing the molecular weaponry required by males to enhance fecundity and drive sexual conflict with females. Some of the key findings are considered in the following sections, since they uncover a complex network of molecular interactions involving several families of proteins found in seminal fluid of all higher eukaryotes.
3.1. Sex Peptide—An Essential Component of the Accessory Gland Secretome
Extensive studies have shown that one molecule, sex peptide (SP or Acp70A [Accessory gland protein 70A]), a 36-amino acid peptide, which is secreted from main cells, is involved in many characterized aspects of female behavioral reprogramming (Chen et al., 1988; Kubli, 2008). SP mutant (or knockdown) males are unable to promote long-term increases in egg laying or reduce female receptivity to remating (Chapman et al., 2003; Chen et al., 1988). Furthermore, they fail to fully induce multiple other postmating changes, including those affecting the innate immune response (Peng, Zipperlen, & Kubli, 2005), hormone and pheromone production (Bontonou, Shaik, Denis, & Wicker-Thomas, 2015; Moshitzky et al., 1996), locomotor activity and sleep (Isaac, Li, Leedale, & Shirras, 2010), food intake (Carvalho, Kapahi, Anderson, & Benzer, 2006; Ribeiro & Dickson, 2010), and excretion (Apger-McGlaughon & Wolfner, 2013; Cognigni, Bailey, & Miguel-Aliaga, 2011).
The long-term postmating activities of SP require the transfer of sperm, the so-called sperm effect (Liu & Kubli, 2003; Manning, 1962). In Drosophila, sperm are stored after mating for at least 1 week in two organs, the paired spermathecae and the seminal receptacle (Fig. 1C). SP binds to sperm in females and is released gradually from the sperm storage organs by protease cleavage to mediate its long-term actions (Peng, Chen, et al., 2005). Wolfner’s group has identified a set of genes expressed by the accessory gland, the long-term response (LTR) network, that is involved in trafficking SP into the sperm storage organs, presumably by permitting its stable binding to sperm (Fig. 2; Findlay et al., 2014; Ravi Ram & Wolfner, 2009). Although some of these protein products enter the female storage organs, they do not appear to persist in females like SP. Interestingly, efficient depletion of sperm from storage organs to fertilize the continuous stream of new eggs produced by the ovaries is dependent on proteolytic release of SP from stored sperm (Avila, Ravi Ram, Bloch Qazi, & Wolfner, 2010). Careful analysis of the effects of SP indicates that it optimizes the male’s short-term fitness benefits, so that on average, he sires more progeny, at least when mating with young females (Fricke, Green, Mills, & Chapman, 2013; Fricke, Wigby, Hobbs, & Chapman, 2009).
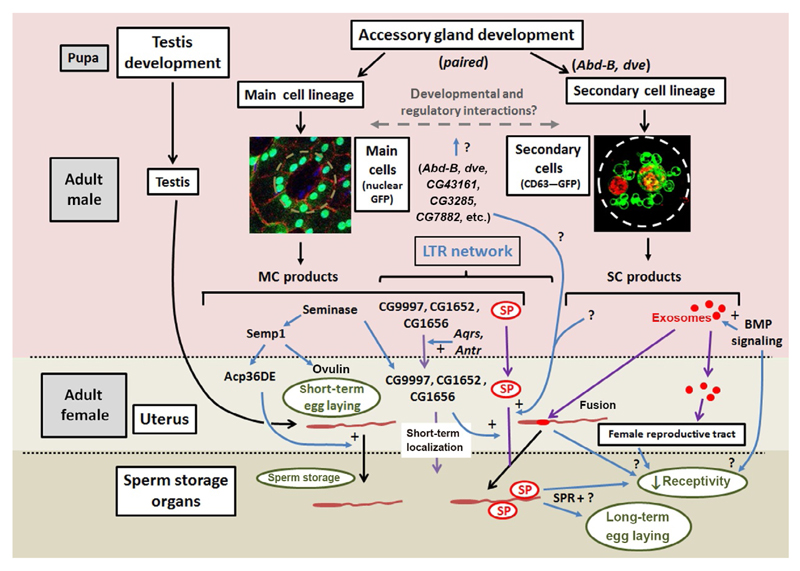
Fig. 2.
Regulation of female postmating responses by the fly accessory gland. Schematic outlines the complex genetic network associated with the accessory glands that regulates female postmating responses in flies. SP and secondary cell exosomes are marked in red, sperm are brown. Key molecular and exosome movements are highlighted with purple arrows, genetic interactions with blue arrows, structural and cellular elements of the reproductive system are in black boxes, and a selected group of postmating responses are also represented (green ellipses). Any developmental defect in secondary cell biology could have indirect actions on main cells that ultimately lead to effects on SP storage in females. Indeed, even adult-specific inhibition of BMP signaling or exosome secretion could affect main cells. However, the transfer of exosomes into females, their subsequent fusion with sperm and selective effect on only some SP-dependent postmating responses, suggests that these vesicles mediate at least some of their effects after mating. Note that the iab-6 mutation in Abd-B leads to defects in glycosylation of main cell proteins such as ovulin. Acp36DE induces uterine contractions and is involved in anterior mating plug formation. Products of the ejaculatory bulb play a key role in forming the posterior mating plug. All these latter effects are not shown here for simplicity.
3.2. Sex Peptide Signaling in Females
Experiments in which mutant forms of SP are expressed in SP null males or where SP or SP mutant peptides are either injected or expressed ectopically in females have demonstrated that SP alone can induce most of the characterized female postmating responses, but that different parts of the molecule are required for different functions (e.g., Chen et al., 1988; Domanitskaya, Liu, Chen, & Kubli, 2007; Tsuda, Peyre, Asano, & Aigaki, 2015). One major breakthrough in our understanding was the identification of a sex peptide receptor (SPR) expressed in females that is required to mediate many of SP’s effects (Yapici, Kim, Ribeiro, & Dickson, 2008). Subsequent work revealed specific SPR-expressing neurons in the female reproductive tract that are necessary and sufficient to induce SP-dependent postmating responses (Häsemeyer et al., 2009; Yang et al., 2009) and some of the circuitry regulated by these neurons (Rezával, Nojima, Neville, Lin, & Goodwin, 2014; Rezával et al., 2012; reviewed in Feng, Palfreyman, Häsemeyer, Talsma, & Dickson, 2014; Kubli & Bopp, 2012; Walker, Corrales-Carvajal, & Ribeiro, 2015). SP is also thought to bind to cells in other parts of the nervous system by entering the hemolymph (Ding, Haussmann, Ottiger, & Kubli, 2003).
Although the SP/SPR model provides a very neat explanation of the accessory gland’s roles in reprogramming long-term female postmating responses, several observations suggest that it cannot represent the whole story. For example, myoinhibitory peptides are alternative ligands for the SPR and are expressed in females, so other aspects of signaling are affected in SPR mutant females (Kim et al., 2010). There is also evidence that SP can induce some postmating responses in females that lack SPR (Haussmann, Hemani, Wijesekera, Dauwalder, & Soller, 2013), suggesting other SP-dependent signaling mechanisms are involved.
3.3. Other Main Cell-Derived Peptides Have Effects on Female Behavior
Importantly, several other main cell-expressed genes have been implicated in inducing short-term female postmating responses (Fig. 2). For example, stimulation of egg laying in the first day postmating is dependent on the seminal fluid proteins ovulin (Acp26Aa; Herndon & Wolfner, 1995) and CG33943 (Ravi Ram & Wolfner, 2007). Another secreted main cell protein, Acp36DE, promotes sperm storage (Bloch Qazi & Wolfner, 2003) and is involved in inducing a series of conformational changes in the uterus both during and immediately after mating that may guide the movement of sperm (Avila & Wolfner, 2009). These functions may be linked to an additional role for Acp36DE in promoting formation of the anterior part of the mating plug, which contains some Acp36DE protein (Bertram et al., 1996). The posterior part of the mating plug is primarily formed from products of the ejaculatory bulb, including the proteins PMBII and PMBme (Avila, Cohen, et al., 2015; Bretman, Lawniczak, Boone, & Chapman, 2010). The mating plug is required to block immediate remating and retain sperm in the female reproductive tract.
Ovulin and Acp36DE must both be cleaved during mating to exert their functions in females. This cleavage is dependent on a protease cascade involving the proenzymes seminase (CG10586; LaFlamme, Ram, & Wolfner, 2012) and Semp1 (CG11864; Ravi Ram, Sirot, & Wolfner, 2006), which are both synthesized by main cells and processed during mating (Fig. 2). Full cleavage of substrates like ovulin only takes place in the female and may require female factors (LaFlamme, Avila, Michalski, & Wolfner, 2014). In addition, seminase regulates a second pathway independently of Semp1 that is required for SP storage on sperm in females, and therefore plays an important role in the long-term female postmating response (LaFlamme et al., 2012). It also appears to affect the transient localization of several other Acps (CG9997, CG1652, CG1656) in the LTR network to the seminal receptacle.
4. Molecular and Functional Parallels between Seminal Fluid Proteins in Flies and Humans
Drosophila genetics has been instrumental in developing a uniquely detailed genetic picture of interacting, main cell-derived molecules secreted into seminal fluid and their reproductive functions. But how relevant to the human prostate or other reproductive glands are the mechanisms involved? One clear similarity between Drosophila and human seminal fluids, which have both been subjected to extensive proteomics analysis, is the presence of specific classes of proteins that appear to have important roles in fertility. For example, members of the cysteine-rich secretory protein (Ernesto et al., 2015; Krätzschmar et al., 1996; Ravi Ram & Wolfner, 2009; Udby et al., 2005) and lectin (Garénaux et al., 2015; Ravi Ram & Wolfner, 2009) families are made by the fly accessory gland and prostate epithelium, and associate directly with sperm or interact with proteins that bind to sperm.
Another feature is the remarkable abundance of proteases and protease inhibitors in seminal fluid (Laflamme & Wolfner, 2013). In flies, at least 20% of identified seminal fluid proteins are proteolysis regulators (Findlay, MacCoss, & Swanson, 2009; Findlay, Yi, Maccoss, & Swanson, 2008). The proteases in humans and flies are characterized into several classes including trypsin- and chymotrypsin-like serine proteases (including kallikreins; some proteases secreted by the accessory gland, like CG4815, share greatest sequence similarity with human kallikrein family members), metalloproteases, cysteine proteases, and aspartic proteases.
4.1. Proteases and Their Inhibitors Have Multiple Functions in Seminal Fluid
In humans, some of the proteases secreted by the seminal vesicles promote coagulation of the ejaculate (Lilja, Oldbring, Rannevik, & Laurell, 1987). The seminal clot that forms is then broken down in a process called liquefaction by a cascade of kallikrein-like serine proteases, including PSA. These proteases are secreted by the prostate and activated when levels of Zn2+ ions fall as seminal fluid components are mixed (Pampalakis & Sotiropoulou, 2007). Liquefaction releases sperm, potentially increasing fertility.
Several other species, including chimpanzees and mice, use an equivalent coagulation mechanism to form a mating plug, which, like its fly equivalent, appears to be involved in reducing successful second matings and in retaining sperm within the female reproductive tract. Knockout studies of a protease inhibitor expressed primarily in the mouse seminal vesicles suggest that regulated proteolytic activity is required to form this plug properly (Murer et al., 2001). In this regard, the activities of the fly accessory gland potentially mirror those of both the seminal vesicles and the prostate in mammals.
Although the requirement for gland-derived protease activity in seminal fluid is well established in both mammals and flies, the precise functional parallels have yet to be fully assessed. Prostate kallikreins can release proteins from the seminal clot that potentially modulate immunity (Emami & Diamandis, 2010), promote inflammation (Sharkey et al., 2012), and inhibit bacterial growth (Edström et al., 2008). Proteins secreted from the Drosophila accessory gland, including SP, also affect female immunity and bacterial resistance (Peng, Zipperlen, et al., 2005; Short, Wolfner, & Lazzaro, 2012); the precise roles of proteases are not yet established, but the activities of multiple proteases are required for SP function. Protease activity is critical for binding of SP to sperm in females (Findlay et al., 2014; LaFlamme et al., 2012), for its subsequent release (Peng, Chen, et al., 2005) and as a result, for the gradual release of sperm from storage (Avila et al., 2010). Sperm storage also takes place to different extents in vertebrates, including humans, who appear to be able to maintain viable sperm for nearly a week. However, the mechanisms involved are poorly characterized (Holt & Fazeli, 2016).
4.2. Rapid Evolution of Seminal Fluid Proteins
One difficulty in comparing the components of seminal fluid in different species, including proteases, is that these molecules often evolve rapidly (Marques et al., 2012; Morrow & Innocenti, 2012; Sirot et al., 2014). This seems to be in part because male interests drive evolutionary changes to maximize offspring and enhance competition with other male mates. The male’s interests may differ from those of the female, leading to conflict between the two sexes and potentially positive selection of specific variants, as is seen, for example, in ovulin orthologues found in different Drosophila species (Fay & Wu, 2000). Just like ovulin, SP orthologues are found in many Drosophila species, but there are no vertebrate equivalents. However, in some mammals, most notably llamas, alpacas, and bulls, seminal fluid contains an ovulation-inducing factor that appears to be equivalent to β-nerve growth factor (Kershaw-Young, Druart, Vaughan, & Maxwell, 2012; Ratto et al., 2012). It could, like SP, function by modulating the female’s nervous system. In rabbits, this seminal protein is primarily expressed by the prostate (Maranesi et al., 2015), although its role in this animal as an ovulation-inducing factor is more controversial (Cervantes, Palomino, & Adams, 2015; Silva et al., 2011).
4.3. Males Strategically Allocate Seminal Fluid Proteins in Reproduction
Studies in Drosophila not only demonstrate that seminal fluid proteins like SP can enhance fitness benefits (Fricke et al., 2009), but also suggest that males can strategically allocate more SP and ovulin to specific females in the presence of a competitor male (Wigby et al., 2009). Even more remarkably, males can alter the composition of seminal fluid when mating with virgin or mated females. Relative to SP, males transfer less ovulin to previously mated females than they do to virgins (Sirot, Wolfner, & Wigby, 2011). SP will inhibit receptivity in both situations, whereas previously mated females will already be laying eggs, so the benefits of ovulin transfer are reduced. How such changes in seminal protein allocations are achieved, when both molecules are synthesized by the same cells primarily before mating begins, remains unclear. But these studies highlight the complex mechanisms by which seminal fluid content can be regulated and its powerful behavioral reprogramming effects that optimize male fecundity and may drive sexual conflict. Generating such a uniquely potent concoction is likely to require specialized adaptations of epithelial secretory mechanisms.
Recent analysis of epithelial cell biology in the fly accessory gland, particularly focusing on the secondary cells in each gland, has revealed some complex secretory and intercellular signaling mechanisms that share surprising parallels to the human prostate epithelium and are required to induce specific postmating responses (Corrigan et al., 2014; Ito et al., 2014; Leiblich et al., 2012).
5. Development and Cellular Organization of the Accessory Gland
5.1. Early Development of the Accessory Gland
There are surprisingly few studies of accessory gland development in the literature. Ahmad and Baker (2002) reported that the gland is derived from mesodermal cells that in male larvae migrate into the genital imaginal disc. The migration requires signaling by the FGF homologue Branchless, which is expressed in the ectodermal imaginal disc; mesodermal cells, which synthesize the FGF receptor Breathless, move toward this signal. In this regard, the accessory gland epithelial layer might appear to parallel the mesoderm-derived seminal vesicles of mammals, rather than the endoderm-derived prostate epithelium (Thomson & Marker, 2006).
The transcription factor Paired (Prd), the founder member of the Pax family, is also essential for growth and proliferation in the accessory glands; when the embryonic lethality of prd mutants is selectively rescued, the resulting adult males are sterile and specifically lack, or have highly reduced, accessory glands (Xue & Noll, 2000, 2002). Interestingly, prd is still expressed in adult main cells and secondary cells, at roughly 100-fold higher levels than any other organ (Chintapalli, Wang, & Dow, 2007). This continued expression appears to be required for normal transcription of seminal protein genes, such as SP and ovulin (Xue & Noll, 2002). As mentioned earlier, specific FGF and Pax genes are involved in prostate development (Schwertfeger, 2009; Xu et al., 2012), but whether this reflects conserved mechanism or the independent deployment of two common developmental regulators in flies and mammals requires a more detailed analysis in both systems.
In fact, at first sight, the adult epithelium of the fly accessory gland has properties that indicate it would not make a good prostate cancer model. Its component cells are all postmitotic, having completed their last mitotic division about halfway through pupation (Taniguchi et al., 2014). About 5–10 h afterward, the cells go through one more mitosis without cytokinesis, so that adult main cells and secondary cells are all binucleate, a phenotype seen in some human tissues, most notably hepatocytes (Grizzi & Chiriva-Internati, 2007) and surface umbrella cells of the bladder transitional epithelium (White, Masters, & Woolf, 1997), but not the prostate. Very little is known about the mechanisms involved in binucleation. But Taniguchi et al. (2014) have shown that Mud, the fly homologue of the cytoskeletal regulator nuclear mitotic apparatus protein (NuMA), which is upregulated in some cancers (Hasholzner et al., 1999; Xavier de Carvalho et al., 2015), is involved in the accessory gland and can indeed induce binucleation in normally mononucleate Drosophila cells. Whether this mechanism is relevant to the binucleate cells observed in some cancers, particularly Hodgkin lymphoma (Farrell & Jarrett, 2011), remains to be tested.
5.2. Developmental Regulation of Secondary Cells
The morphology of adult secondary cells, which contain many large intracellular compartments involved in secretion (Fig. 3; Corrigan et al., 2014; Rylett, Walker, Howell, Shirras, & Isaac, 2007), is very different from main cells (Bairati, 1968). Recent studies have identified at least two transcriptional regulators involved in secondary cell formation, differentiation, and/or survival. The homeodomain transcriptional repressor Defective proventriculus (Dve) is expressed at high levels in secondary cells by mid-pupation, and only weakly in main cells (Minami et al., 2012). This strong secondary cell expression persists in adults. Loss of dve function reduces the number of secondary cells in the accessory gland, a phenotype that seems to involve some cell death (Minami et al., 2012). Those secondary cells that remain are small and lack large secretory compartments. dve also appears to play a role in main cell binucleation.
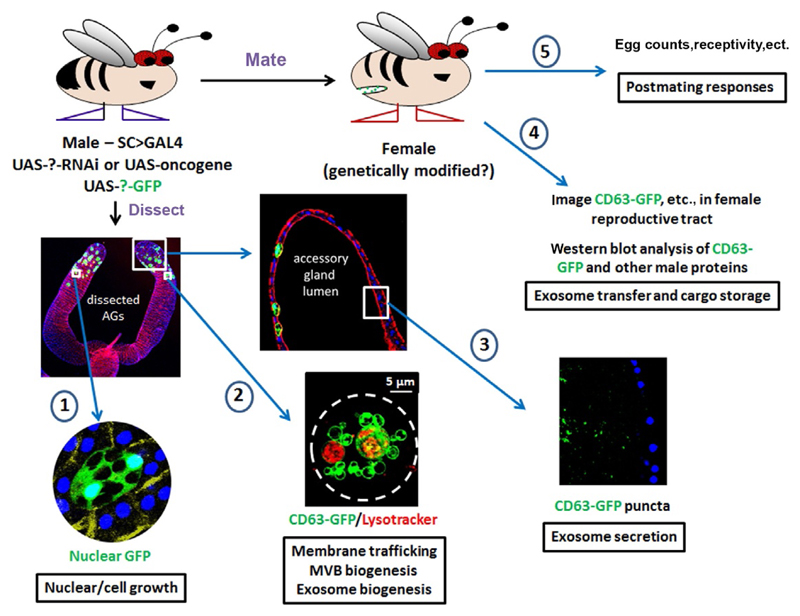
Fig. 3.
The secondary cell as a versatile in vivo model to study endolysosomal trafficking, exosome biogenesis, and functions. Diagram illustrates the different approaches that can be employed to study processes associated with exosome biogenesis and functions using the fly accessory glands, ranging from analysis of growth (1) and membrane trafficking/vesicle formation (2) in SCs, to exosome secretion into the gland lumen (3), to analysis of exosome transfer (4), and effects on postmating responses (5) in females. The living secondary cell shown in (2) is marked with CD63-GFP and stained with Lysotracker Red to reveal large acidic compartments, which, as in this case, are typically greater than 5 μm in diameter in older males. Note that the male and female can both be genetically modified in different ways to affect exosome-secreting and target cells, respectively. Exosomes and the compartments that produce them can also be imaged by more standard approaches, such as transmission electron microscopy (Corrigan et al., 2014).
The Hox gene Abd-B is expressed specifically in secondary cells within the adult accessory gland, and mutations affecting the iab-6 enhancer in this gene, which can independently drive secondary cell-specific reporter gene expression, lead to loss of this cell’s characteristic secretory morphology (Gligorov, Sitnik, Maeda, Wolfner, & Karch, 2013). Importantly, dve and Abd-B are both required to induce long-term egg laying in mated females and to suppress receptivity to other males (Gligorov et al., 2013; Minami et al., 2012). Therefore, secondary cells appear to provide factors essential for long-term female postmating responses that function together with SP and other main cell products (Fig. 2). Females that mate with iab-6 mutant males fail to store SP normally in their seminal receptacle (Gligorov et al., 2013). Furthermore, several main cell-derived Acps, including ovulin, are not normally glycosylated in the accessory gland. Whether this reflects an indirect effect of secondary cells on the glycosylation capacity of main cells, a secondary cell-specific modification of Acps in the accessory gland lumen, or an ability of secondary cells to take up main cell products and modify them remains unclear.
5.3. Abd-B-Regulated Genes Control Secondary Cell Functions
A recent transcriptomics screen of genes expressed by the accessory glands of wild-type and iab-6 mutant males identified 77 genes that are downregulated by at least fivefold and 115 genes that are at least fivefold upregulated (Sitnik, Gligorov, Maeda, Karch, & Wolfner, 2016). Interestingly, some genes like CG11598, which are expressed at high levels in both main cells and secondary cells, are strongly downregulated in mutant glands, while other genes encoding secreted proteins are highly upregulated. This is consistent with the idea that in development and/or in adults, there are major regulatory interactions between secondary cells and main cells (Fig. 2). Knockdown in secondary cells of some of the genes requiring Abd-B for their expression (CG7882, CG9509, and CG14069), using an iab-6-GAL4 driver, produces cellular phenotypes where large secretory compartments are absent or small, and also leads to a suppression of postmating responses (egg laying and receptivity changes) and reduced SP storage in mated females (Sitnik et al., 2016). However, knockdown of other genes that are downregulated in Abd-B mutant glands (e.g., CG43161, CG3285) affects these same postmating responses, but does not produce obvious cellular phenotypes.
All these studies highlight important functions for secondary cells in the induction of postmating responses and SP storage. However, because they employ knockdown throughout accessory gland development, they could be explained by defects that are generated during pupation, potentially altering the development of secondary cells, and indirectly affecting the development of main cells or other cells. Experiments focused only on the adult have revealed more specific roles for these cells in postmating responses and some prostate-like cell biological properties.
6. Functions of the Adult Secondary Cell
6.1. Aging Adult Secondary Cells Continue to Grow
Morphological analysis of the aging adult accessory gland reveals that unlike main cells, which become more squamous as the glandular lumen expands, secondary cells enlarge in nuclear and total cell volume (Leiblich et al., 2012). Although nuclei in some cell types enlarge by endoduplication (Zielke, Edgar, & DePamphilis, 2013), secondary cell nuclei do not appear to replicate their DNA as they grow, mirroring several other cell types in flies that alter their nuclear size in response to growth signals in the absence of DNA synthesis (Gao & Pan, 2001). Interestingly, secondary cell growth is affected by sexual activity, accelerating in multiply-mated males.
6.2. BMP Signaling Controls Adult Secondary Cell Growth and Migration
Cell type-specific expression is most commonly achieved in Drosophila using the GAL4/UAS modular misexpression system (Brand & Perrimon, 1993). By employing a ubiquitously expressed, temperature-sensitive form of the GAL4 antagonist, GAL80, GAL4-induced expression can be activated only in adults by shifting the culture temperature to 29°C at eclosion (McGuire, Mao, & Davis, 2004). Using this approach, Leiblich et al. (2012) demonstrated that adult secondary cell growth requires BMP signaling. Blocking BMP signaling in these cells has no effect on female egg laying, but does suppress female receptivity to remating, indicating that these two processes, which both normally require stored SP, are differentially controlled by adult male-specific, secondary cell-dependent factors (Fig. 2). One alternative explanation is that other seminal proteins like ovulin act independently of SP to promote long-term effects on ovulation. However, this seems relatively unlikely, since such a mechanism could only work in this specific scenario, because these factors cannot induce long-term ovulation in the absence of SP or when SCs fail to develop normally (Gligorov et al., 2013; Minami et al., 2012).
Surprisingly, if aging males mate, a small subset of secondary cells also sporadically delaminates from the apical surface of the epithelium in a BMP-dependent fashion (Leiblich et al., 2012). These cells migrate along the epithelium to the proximal end of the gland and are transferred to females upon mating. This transfer is not essential for normal fertility, because it does not occur in young males or following every mating in older males. One possibility is that in those females that receive secondary cells, the cells continue to secrete into the uterus prior to egg laying and increase the potency of the aging ejaculate. Alternatively, these cells may be a by-product of the BMP-mediated events required to suppress female receptivity, which occur in secondary cells that remain embedded in the glandular epithelium.
6.3. BMP Signaling in the Human Prostate
BMP signaling is involved in prostate growth and development (Omori et al., 2014). It has growth-inhibitory (Ding et al., 2011) and -stimulatory (Lee et al., 2011) effects in prostate cancer and has been implicated in promoting hormone resistance in bone metastases (Lee et al., 2014). Prostate epithelial cells are also found in seminal fluid (Barren et al., 1998), though it has been assumed they slough off and are not the product of an active delamination process. Ito et al. (2014) have used the secondary cell system to screen for genes that regulate migration and shown that identified candidate genes, which are evolutionarily conserved, also affect invasive properties of prostate cancer cells, although not in the way that the screen might have predicted. To better understand the roles of BMP signaling in secondary cells, Corrigan et al. (2014) undertook a more detailed cell biological analysis to characterize cell biological defects associated with altered signaling.
6.4. Secretion by Adult Secondary Cells
There are about 15 large (≥3 μm in diameter) intracellular compartments in 3-day-old adult secondary cells, most of which contain dense-core granules (DCGs; Rylett et al., 2007). Such granules are present in many higher eukaryotic cells involved in regulated secretion, like pancreatic beta-cells, and store bioactive small molecules, peptides, and proteins, as well as proteases that can cleave proteins into active forms (Kim, Gondré-Lewis, Arnaoutova, & Loh, 2006). The bioactive molecules in secondary cell DCGs are not known. However, the fly homologue of angiotensin-I-converting enzyme, which is also synthesized by prostate epithelial cells and appears to be linked to prostate cancer susceptibility (Nassis et al., 2001; Xie, You, & Chen, 2014), is present in these granules (Rylett et al., 2007).
6.5. Secondary Cells Secrete Exosomes That Inhibit Female Receptivity
Using live confocal imaging with different YFP-tagged Rab GTPases, which are conserved markers of specific membrane-bound compartments in all eukaryotic cells (Stenmark, 2009), Corrigan et al. (2014) demonstrated that the DCG-containing compartments are marked by the recycling endosome marker Rab11. However, about three other large compartments have acidic lumens and are marked by Rab7, identifying them as late endosomes or lysosomes (Fig. 3). When a GFP-tagged form of the human transmembrane exosome marker CD63 is expressed in these cells, almost all of the large compartments are marked, and GFP puncta are observed inside the large acidic compartments and in the lumen of the gland. Transmission electron microscopy confirms the presence of vesicles in these compartments and the lumen, indicating that the GFP-labeled structures are exosomes. Luminal puncta can be counted to measure levels of exosome secretion (Fig. 3).
The CD63-GFP-positive exosomes are transferred to females upon mating, where they fuse with sperm and appear to interact with the lining of the female reproductive tract (Corrigan et al., 2014). Multiple genetic manipulations that knock down conserved genes involved in human exosome biogenesis, including members of the ESCRT (endosomal sorting complexes required for transport) family, which control formation of intraluminal vesicles in MVBs, and Rabs, which regulate endosomal compartment trafficking to the cell surface (Colombo et al., 2014), suppress exosome secretion from adult secondary cells. Remarkably, these manipulations also prevent males from fully inhibiting female receptivity after mating, but do not affect the induction of egg laying, potentially highlighting a selective role in modifying specific female postmating behaviors.
Further analysis revealed that BMP signaling is absolutely required for exosome secretion and controls membrane trafficking through the endolysosomal system, suggesting that its effects on female receptivity may be mediated by exosomes (Corrigan et al., 2014). Since the long-term effects of mating on egg laying are retained in the absence of exosomes, SP is likely to still bind to stored sperm. Hence, these findings indicate that BMP-dependent exosomes are either involved in regulating a specific aspect of SP function or that they act in a parallel pathway that selectively influences female receptivity (Fig. 2). Interestingly, by expressing SP in specific neurons of wild-type and SPR mutant females, Haussmann et al. (2013) have also provided evidence that egg laying and receptivity are not regulated by identical neural circuits or signaling cascades, supporting the hypothesis that SP mediates its actions via more than one downstream pathway.
6.6. Secondary Cell Exosomes and Prostasomes in Fertility and Sexual Conflict
Prostasomes from human prostate epithelial cells also target sperm, at least in vitro, and have been reported to deliver several molecules that promote motility and capacitation (Park et al., 2011), although this remains controversial (Pons-Rejraji et al., 2011). They may also coat the sperm surface and modulate the female immune response to sperm (Ronquist, 2015). In flies, the absence of secondary cell exosomes does not significantly affect the number of offspring produced by sperm, but does suppress a postmating behavioral effect that involves interaction between sperm and female tissues, namely, female receptivity. Whether these exosomes fuse with and directly reprogramme female cells in addition to sperm remains unclear. However, such a mechanism would provide a novel weapon to drive sexual conflict by potentially delivering intracellular signaling components, miRNAs, and membrane-bound receptors to overcome cellular processes that favor female interests. Several studies (reviewed in Sirot et al., 2009) reveal a complex regulation of female responses after mating, not just affecting behaviors and metabolism, but also modulating the female reproductive tract directly. The latter changes involve multiple male signals and include release of neurotransmitters from female nerve termini (e.g., Heifetz, Lindner, Garini, & Wolfner, 2014) and activation of signaling in sperm storage organs (Avila, Mattei, & Wolfner, 2015). It will be interesting to test which, if any, of these responses are exosome dependent.
Progesterone receptors and Ca2+ signaling machinery have been shown to be transferred to human sperm by prostasomes (Park et al., 2011), providing a means of rapidly upregulating specific signaling cascades in sperm after mating. However, the active molecules in secondary cell exosomes are yet to be identified. Males of another Drosophila species, Drosophila mojavensis, transfer mRNAs from the accessory gland to females (Bono, Matzkin, Kelleher, & Markow, 2011), though their cellular origin is unknown. The levels of RNA or proteins transferred by exosomes are almost certainly low, and probably only the most abundant will be detectable by standard transcriptomics or proteomics techniques unless large quantities of exosomes can be isolated. A major debate in the exosome field is how exosomes can deliver sufficiently high levels of bioactive molecules to alter cell behavior under physiological conditions (e.g., Chevillet et al., 2014). The genetic tractability of the fly system should allow this conundrum to be resolved, and provide the opportunity to independently manipulate exosome-secreting and potential target cells (in males and females, respectively) to dissect out the mechanisms involved (Fig. 3).
7. Modeling Prostate Cancer Biology in Secondary Cells
7.1. Studying Exosome Regulation and Functions in Flies
A flurry of recent reports has implicated exosomes and other secreted extracellular vesicles in multiple aspects of cancer biology, including modulation of the tumor microenvironment, transfer of drug resistance and malignant cell properties, immunosuppression, and priming and establishment of premetastatic sites (e.g., Costa-Silva et al., 2015; Hoshino et al., 2015; Ji et al., 2015; Peinado et al., 2012; Zhang et al., 2015; Zhou et al., 2014; Zomer et al., 2015). If exosomes from human male reproductive glands are involved in modulating female physiology and cell function, as they are in flies, it is perhaps not surprising that when they are released at high levels into the male’s circulation by prostate cancer cells, which have lost their polarity, they might mediate multiple cancer-promoting effects on some target cells.
As discussed earlier, seminal fluid proteins with roles in reproduction are not highly evolutionarily conserved, but the cellular mechanisms controlling exosome biogenesis and secretion are (Corrigan et al., 2014). Perhaps the greatest challenge in current exosome research is to understand the detailed cell biology of exosome biogenesis. Both ESCRT-dependent (Baietti et al., 2012) and -independent (Stuffers, Sem Wegner, Stenmark, & Brech, 2009; Trajkovic et al., 2008) mechanisms have been proposed, and a number of different Rab GTPases appear to control the process of secretion either coordinately or independently in different cell types (Colombo et al., 2014). Intraluminal vesicles and the compartments in which they are formed are typically at the limit of fluorescence microscopy resolution, so most studies rely on electron microscopy to visualize these structures. Alternatively the compartments are artificially enlarged by genetic manipulation with a constitutively activated form of the early endosomal regulator Rab5 (Baietti et al., 2012; Trajkovic et al., 2008), a treatment that inevitably alters the identity of MVBs.
The substructure of multivesicular endosomes and lysosomes, and the generation and dynamics of intraluminal vesicles can be visualized in real time by confocal microscopy in secondary cells (Corrigan et al., 2014), overcoming this hurdle. This system should allow some of the key questions in exosome biology to be addressed: For example, are intraluminal vesicles loaded with different cargos in the same or different compartments? Do these vesicles traffic differently out of the cell? What distinguishes vesicles targeted for degradation in the lysosome from those destined to be secreted? Answers to all these questions will not only be relevant to prostate cancer but also to the biology of many other cancers, where exosome biogenesis mechanisms are poorly characterized.
7.2. Signaling and Exosome Biogenesis
The discovery that BMP signaling plays such a critical role in exosome secretion from Drosophila SCs already highlights a strong connection between intracellular signaling and its control of intercellular exosome-mediated communication. With defective intracellular signaling playing such a key role in cancer biology, dissecting out links of this kind is likely to inform our understanding of how tumors modulate their microenvironment. It may also indicate new ways of extending the use of exosomes for cancer diagnostics (e.g., Melo et al., 2015), employing emerging high-throughput techniques (He, Crow, Roth, Zeng, & Godwin, 2014) to detect specific signaling signatures or responses to drugs. Indeed, the complex roles of BMP signaling in prostate cancer (Ding et al., 2011; Lee et al., 2011, 2014) need to be reevaluated in the context of its possible effects on exosome regulation.
7.3. Growth and Exosome Biogenesis
The enlarged late endosomes and lysosomes of secondary cells also illustrate the importance of endocytic trafficking in cells adapted to high level exosome secretion (Corrigan et al., 2014). These same compartments are required for amino acid-dependent activation of the growth and metabolic regulator, mechanistic target of rapamycin complex 1 (mTORC1; Bar-Peled & Sabatini, 2014; Goberdhan, 2010; Goberdhan, Wilson, & Harris, 2016), which plays a central role in cancer. This may provide an explanation for the observation that cancer cells, in which mTORC1 signaling is frequently upregulated, often secrete increased numbers of exosomes. And since mTORC1 signaling itself controls endolysosomal trafficking (Kim et al., 2015; Peña-Llopis & Brugarolas, 2011), it reveals a complex link between growth signaling and exosome secretion that requires further analysis.
7.4. Steroid Signaling in the Male Reproductive System
One critical aspect of prostate biology that cannot be fully modeled in fly accessory glands is androgen signaling. However, the major steroid hormone in Drosophila, ecdysone, which has primarily been characterized as a regulator of developmental transitions (reviewed in Yamanaka, Rewitz, & O’Connor, 2013), has more recently been shown to be regulated by the socio-sexual environment of adult males (Ganter et al., 2011; Ishimoto, Sakai, & Kitamoto, 2009; Schwedes & Carney, 2012). It also affects somatic and germline stem cell maintenance in the testis (Li, Ma, Cherry, & Matunis, 2014; Qian et al., 2014) and accessory gland secretion (Wolfner et al., 1997). Since male flies can alter the levels of different seminal fluid proteins in response to the flies around them (Fricke et al., 2009; Wigby et al., 2009), it is appealing to speculate that ecdysone signaling might provide a link between a male’s environment and accessory gland secretion. In fact, Hentze et al. (2013) have suggested that secondary cells might act as a source of ecdysone. Studying both ecdysone synthesis and the possible roles of the ecdysone receptor, which shares broad structural similarity to the AR, in the accessory gland are important goals in the immediate future.
7.5. The Secondary Cell as a General Model for Exosome Biology
Analysis of exosome biogenesis in the fly system is likely to also inform our understanding of exosome control in other normal and cancer cell types. Even the idea that exosomes are secreted to reprogramme another individual is not unique to male reproductive glands. Exosomes are abundant in breast milk and are proposed to play roles in modulating immunity in newborn babies (Melnik, John, & Schmitz, 2014). Furthermore, visualizing exosome biogenesis in living cells and observing the specific changes that take place when these cells are genetically manipulated will also reveal whether these vesicles play other roles inside cells and how these coordinate with their extracellular signaling functions. Therefore, despite the diminutive size of the fly and its accessory gland, the 40 secondary cells and their enlarged vesicle-containing compartments offer a new way to address some of the most challenging questions in cancer biology and potentially a direct link to prostate subcellular biology.
Acknowledgments
We apologize to those authors whose articles we were not able to cite because of space limitations. We thank Sumeth Perera for helpful comments on the manuscript. The authors gratefully acknowledge the support of the BBSRC (BB/K017462/1, BB/L007096/1), Cancer Research UK (C191591/A6181, C19591/A9093, C7713/A6174, C19591/A19076), C38302/A12278 grants through the Cancer Research UK Oxford Centre Development Fund, and the John Fell Fund, Oxford, as well as studentships and scholarships from the Wellcome Trust, CRUK, Urology Foundation, and the MRC in developing their research in this area.
References
- Aalberts M, Stout TA, Stoorvogel W. Prostasomes: Extracellular vesicles from the prostate. Reproduction. 2013;147:R1–R14. doi: 10.1530/REP-13-0358. [DOI] [PubMed] [Google Scholar]
- Ahmad SM, Baker BS. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell. 2002;109:651–661. doi: 10.1016/s0092-8674(02)00744-4. [DOI] [PubMed] [Google Scholar]
- Apger-McGlaughon J, Wolfner MF. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. Journal of Insect Physiology. 2013;59:1024–1030. doi: 10.1016/j.jinsphys.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. pii, S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- Aumüller G, Riva A. Morphology and functions of the human seminal vesicle. Andrologia. 1992;24:183–196. doi: 10.1111/j.1439-0272.1992.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Avila FW, Cohen AB, Ameerudeen FS, Duneau D, Suresh S, Mattei AL, et al. Retention of ejaculate by Drosophila melanogaster females requires the male-derived mating plug protein PEBme. Genetics. 2015a;200:1171–1179. doi: 10.1534/genetics.115.176669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Mattei AL, Wolfner MF. Sex peptide receptor is required for the release of stored sperm by mated Drosophila melanogaster females. Journal of Insect Physiology. 2015b;76:1–6. doi: 10.1016/j.jinsphys.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 2010;186:595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15796–15800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature Cell Biology. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Bairati A. Structure and ultrastructure of the male reproductive system in Drosophila melanogaster: The genital duct and accessory glands. Monitore Zoologico Italiano. 1968;2:105–182. [Google Scholar]
- Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends in Cell Biology. 2014;24:400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barren RJ, 3rd, Holmes EH, Boynton AL, Gregorakis A, Elgamal AA, Cobb OE, et al. Method for identifying prostate cells in semen using flow cytometry. Prostate. 1998;36:181–188. doi: 10.1002/(sici)1097-0045(19980801)36:3<181::aid-pros6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bell GP, Thompson BJ. Colorectal cancer progression: Lessons from Drosophila? Seminars in Cell & Developmental Biology. 2014;28:70–77. doi: 10.1016/j.semcdb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. The Journal of Urology. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- Bertram MJ, Akerkar GA, Ard RL, Gonzalez C, Wolfner MF. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mechanisms of Development. 1992;38:33–40. doi: 10.1016/0925-4773(92)90036-j. [DOI] [PubMed] [Google Scholar]
- Bertram MJ, Neubaum DM, Wolfner MF. Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochemistry and Molecular Biology. 1996;26:971–980. doi: 10.1016/s0965-1748(96)00064-1. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. The Journal of Experimental Biology. 2003;206:3521–3528. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- Bono JM, Matzkin LM, Kelleher ES, Markow TA. Postmating transcriptional changes in reproductive tracts of con- and heterospecifically mated Drosophila mojavensis females. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7878–7883. doi: 10.1073/pnas.1100388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontonou G, Shaik HA, Denis B, Wicker-Thomas C. Acp70A regulates Drosophila pheromones through juvenile hormone induction. Insect Biochemistry and Molecular Biology. 2015;56:36–49. doi: 10.1016/j.ibmb.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bretman A, Lawniczak MK, Boone J, Chapman T. A mating plug protein reduces early female remating in Drosophila melanogaster. Journal of Insect Physiology. 2010;56:107–113. doi: 10.1016/j.jinsphys.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2200–2205. doi: 10.1073/pnas.1305609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess J, Del Bel LM, Ma CI, Barylko B, Polevoy G, Rollins J, et al. Type II phosphatidylinositol 4-kinase regulates trafficking of secretory granule proteins in Drosophila. Development. 2012;139:3040–3050. doi: 10.1242/dev.077644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess J, Jauregui M, Tan J, Rollins J, Lallet S, Leventis PA, et al. AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Molecular Biology of the Cell. 2011;22:2094–2105. doi: 10.1091/mbc.E11-01-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JA. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14301–14306. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Research UK. Cancer statistics. 2012 http://www.cancerresearchuk.org/content/cancer-statistics-for-the-uk.
- Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Current Biology. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes MP, Palomino JM, Adams GP. In vivo imaging in the rabbit as a model for the study of ovulation-inducing factors. Laboratory Animals. 2015;49:1–9. doi: 10.1177/0023677214547406. [DOI] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, et al. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Böhlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nature Genetics. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nature Reviews Urology. 2009;6:429–439. doi: 10.1038/nrurol.2009.127. [DOI] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metabolism. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Corrigan L, Redhai S, Leiblich A, Fan S-J, Perera SMW, Patel R, et al. BMP-regulated exosomes from Drosophila male reproductive glands reprogramme female behaviour. The Journal of Cell Biology. 2014;206:671–688. doi: 10.1083/jcb.201401072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature Cell Biology. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das TK, Cagan RL. A Drosophila approach to thyroid cancer therapeutics. Drug Discovery Today Technologies. 2013;10:e65–e71. doi: 10.1016/j.ddtec.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declan JP, Cahill DJP, Chandra A, Davies C. Bladder, prostate and urethra. In: Standring S, Healy J, editors. Gray’s anatomy. Amsterdam: Elsevier; 2009. pp. 2280–2311. [Google Scholar]
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Haussmann I, Ottiger M, Kubli E. Sex-peptides bind to two molecularly different targets in Drosophila melanogaster females. Journal of Neurobiology. 2003;55:372–384. doi: 10.1002/neu.10218. [DOI] [PubMed] [Google Scholar]
- Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanitskaya EV, Liu H, Chen S, Kubli E. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. The FEBS Journal. 2007;274:5659–5668. doi: 10.1111/j.1742-4658.2007.06088.x. [DOI] [PubMed] [Google Scholar]
- Duff J, McEwan IJ. Mutation of histidine 874 in the androgen receptor ligand-binding domain leads to promiscuous ligand activation and altered p160 coactivator interactions. Molecular Endocrinology. 2005;19:2943–2954. doi: 10.1210/me.2005-0231. [DOI] [PubMed] [Google Scholar]
- Edgren G, Adami HO, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406–1414. doi: 10.1136/gutjnl-2012-302412. [DOI] [PubMed] [Google Scholar]
- Edström AM, Malm J, Frohm B, Martellini JA, Giwercman A, Mörgelin M, et al. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. Journal of Immunology. 2008;181:3413–3421. doi: 10.4049/jimmunol.181.5.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N, Diamandis EP. Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGF beta 1 in semen. Biological Chemistry. 2010;391:85–95. doi: 10.1515/BC.2010.007. [DOI] [PubMed] [Google Scholar]
- Ernesto JI, Weigel Muñoz M, Battistone MA, Vasen G, Martínez-López P, Orta G, et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. The Journal of Cell Biology. 2015;210:1213–1224. doi: 10.1083/jcb.201412041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell K, Jarrett RF. The molecular pathogenesis of Hodgkin lymphoma. Histopathology. 2011;58:15–25. doi: 10.1111/j.1365-2559.2010.03705.x. [DOI] [PubMed] [Google Scholar]
- Fay JC, Wu CI. Hitchhiking under positive Darwinian selection. Genetics. 2000;155:1405–1413. doi: 10.1093/genetics/155.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K, Palfreyman MT, Häsemeyer M, Talsma A, Dickson BJ. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron. 2014;83:135–148. doi: 10.1016/j.neuron.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Findlay GD, MacCoss MJ, Swanson WJ. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Research. 2009;19:886–896. doi: 10.1101/gr.089391.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Sitnik JL, Wang W, Aquadro CF, Clark NL, Wolfner MF. Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PLoS Genetics. 2014;10:e1004108. doi: 10.1371/journal.pgen.1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biology. 2008;6:e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser LR. Fertilisation promoting peptide: A key player in male fertility/sub-fertility? Expert Opinion on Investigational Drugs. 1997;6:1797–1801. doi: 10.1517/13543784.6.12.1797. [DOI] [PubMed] [Google Scholar]
- Fricke C, Green D, Mills WE, Chapman T. Age-dependent female responses to a male ejaculate signal alter demographic opportunities for selection. Proceedings of the Biological Sciences. 2013;280:20130428. doi: 10.1098/rspb.2013.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke C, Wigby S, Hobbs R, Chapman T. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. Journal of Evolutionary Biology. 2009;22:275–286. doi: 10.1111/j.1420-9101.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- Ganter GK, Panaitiu AE, Desilets JB, Davis-Heim JA, Fisher EA, Tan LC, et al. Drosophila male courtship behavior is modulated by ecdysteroids. Journal of Insect Physiology. 2011;57:1179–1184. doi: 10.1016/j.jinsphys.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes & Development. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garénaux E, Kanagawa M, Tsuchiyama T, Hori K, Kanazawa T, Goshima A, et al. Discovery, primary, and crystal structures and capacitation-related properties of a prostate-derived heparin-binding protein WGA16 from boar sperm. The Journal of Biological Chemistry. 2015;290:5484–5501. doi: 10.1074/jbc.M114.635268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist AS, Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: The relation between sperm transfer and copulation duration. Evolution. 2000;54:534–542. doi: 10.1111/j.0014-3820.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Research. 1997;57:4687–4691. [PubMed] [Google Scholar]
- Gligorov D, Sitnik JL, Maeda RK, Wolfner MF, Karch F. A novel function for the Hox gene Abd-B in the male accessory gland regulates the long-term female post-mating response in Drosophila. PLoS Genetics. 2013;9:e1003395. doi: 10.1371/journal.pgen.1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC. Intracellular amino acid sensing and mTORC1-regulated growth: New ways to block an old target? Current Opinion in Investigational Drugs. 2010;11:1360–1367. [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C, Harris AL. Amino acid sensing by mTORC1: Intracellular transporters mark the spot. Cell Metabolism. 2016;23:580–589. doi: 10.1016/j.cmet.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni D, Froldi F, Pession A. Connecting epithelial polarity, proliferation and cancer in Drosophila: The many faces of lgl loss of function. The International Journal of Developmental Biology. 2013;57:677–687. doi: 10.1387/ijdb.130285dg. [DOI] [PubMed] [Google Scholar]
- Grizzi F, Chiriva-Internati M. Human binucleate hepatocytes: Are they a defence during chronic liver diseases? Medical Hypotheses. 2007;69:258–261. doi: 10.1016/j.mehy.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Häsemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Hasholzner U, Stieber P, Zimmermann A, Burges A, Hofmann K, Schmitt UM, et al. Nuclear mitotic apparatus protein (NuMA) in benign and malignant diseases. Anticancer Research. 1999;19:2415–2420. [PubMed] [Google Scholar]
- Haussmann IU, Hemani Y, Wijesekera T, Dauwalder B, Soller M. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proceedings of the Biological Sciences. 2013;280:20131938. doi: 10.1098/rspb.2013.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SW, Cunha GR. The prostate: Development and physiology. Radiologic Clinics of North America. 2000;38:1–14. doi: 10.1016/s0033-8389(05)70146-9. [DOI] [PubMed] [Google Scholar]
- He M, Crow J, Roth M, Zeng Y, Godwin AK. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab on a Chip. 2014;14:3773–3780. doi: 10.1039/c4lc00662c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Lindner M, Garini Y, Wolfner MF. Mating regulates neuro-modulator ensembles at nerve termini innervating the Drosophila reproductive tract. Current Biology. 2014;24:731–737. doi: 10.1016/j.cub.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze JL, Moeller ME, Jørgensen AF, Bengtsson MS, Bordoy AM, Warren JT, et al. Accessory gland as a site for prothoracicotropic hormone controlled ecdysone synthesis in adult male insects. PLoS One. 2013;8:e55131. doi: 10.1371/journal.pone.0055131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Wolfner MF. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt WV, Fazeli A. Sperm storage in the female reproductive tract. Annual Review of Animal Biosciences. 2016;4:291–310. doi: 10.1146/annurev-animal-021815-111350. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology. 2012;60:59–74. doi: 10.1111/j.1365-2559.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proceedings of the Biological Sciences. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Sakai T, Kitamoto T. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6381–6386. doi: 10.1073/pnas.0810213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Ueda T, Ueno A, Nakagawa H, Taniguchi H, Kayukawa N, et al. A genetic screen in Drosophila for regulators of human prostate cancer progression. Biochemical and Biophysical Research Communications. 2014;451:548–555. doi: 10.1016/j.bbrc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, et al. Animal models of human prostate cancer: The consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Research. 2013;73:2718–2736. doi: 10.1158/0008-5472.CAN-12-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Sarmad R, Arora S, Dasaraju R, Sarmad K. Prostate cancer for the internist. North American Journal of Medical Sciences. 2015;7:429–435. doi: 10.4103/1947-2714.168660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb JM, DiBenedetto AJ, Wolfner MF. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8093–8097. doi: 10.1073/pnas.90.17.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw-Young CM, Druart X, Vaughan J, Maxwell WM. β-Nerve growth factor is a major component of alpaca seminal plasma and induces ovulation in female alpacas. Reproduction Fertility and Development. 2012;24:1093–1097. doi: 10.1071/RD12039. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Bartalska K, Audsley N, Yamanaka N, Yapici N, Lee JY, et al. MIPs are ancestral ligands for the sex peptide receptor. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6520–6525. doi: 10.1073/pnas.0914764107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology (Bethesda) 2006;21:124–133. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- Kim YM, Jung CH, Seo M, Kim EK, Park JM, Bae SS, et al. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Molecular Cell. 2015;57:207–218. doi: 10.1016/j.molcel.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusza S, Deng WM. At the crossroads of differentiation and proliferation: Precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. Bioessays. 2011;33:124–134. doi: 10.1002/bies.201000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krätzschmar J, Haendler B, Eberspaecher U, Roosterman D, Donner P, Schleuning WD. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. European Journal of Biochemistry. 1996;236:827–836. doi: 10.1111/j.1432-1033.1996.t01-1-00827.x. [DOI] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: Seminal peptides of the Drosophila male. Cellular and Molecular Life Sciences. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. Sexual behaviour: A receptor for sex control in Drosophila females. Current Biology. 2008;18:R210–R212. doi: 10.1016/j.cub.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Kubli E, Bopp D. Sexual behavior: How sex peptide flips the postmating switch of female flies. Current Biology. 2012;22:R520–R522. doi: 10.1016/j.cub.2012.04.058. [DOI] [PubMed] [Google Scholar]
- LaFlamme BA, Avila FW, Michalski K, Wolfner MF. A Drosophila protease cascade member, seminal metalloprotease-1, is activated stepwise by male factors and requires female factors for full activity. Genetics. 2014;196:1117–1129. doi: 10.1534/genetics.113.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme BA, Ram KR, Wolfner MF. The Drosophila melanogaster seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genetics. 2012;8:e1002435. doi: 10.1371/journal.pgen.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme BA, Wolfner MF. Identification and function of proteolysis regulators in seminal fluid. Molecular Reproduction and Development. 2013;80:80–101. doi: 10.1002/mrd.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb AD, Massie CE, Neal DE. The transcriptional programme of the androgen receptor (AR) in prostate cancer. BJU International. 2014;113:358–366. doi: 10.1111/bju.12415. [DOI] [PubMed] [Google Scholar]
- Lee YC, Cheng CJ, Bilen MA, Lu JF, Satcher RL, Yu-Lee LY, et al. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Research. 2011;71:5194–5203. doi: 10.1158/0008-5472.CAN-10-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GT, Kang DI, Ha YS, Jung YS, Chung J, Min K, et al. Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. British Journal of Cancer. 2014;110:1634–1644. doi: 10.1038/bjc.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiblich A, Marsden L, Gandy C, Corrigan L, Jenkins R, Hamdy F, et al. Bone morphogenetic protein- and mating-dependent secretory cell growth and migration in the Drosophila accessory gland. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19292–19297. doi: 10.1073/pnas.1214517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation. 2008;76:699–716. doi: 10.1111/j.1432-0436.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:2773–2780. doi: 10.1158/1055-9965.EPI-07-0546. [DOI] [PubMed] [Google Scholar]
- Li Y, Ma Q, Cherry CM, Matunis EL. Steroid signaling promotes stem cell maintenance in the Drosophila testis. Developmental Biology. 2014;394:129–141. doi: 10.1016/j.ydbio.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H, Oldbring J, Rannevik G, Laurell CB. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. The Journal of Clinical Investigation. 1987;80:281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RM, Morrissey C, Fitzpatrick JM, Watson RW. Prostate epithelial cell differentiation and its relevance to the understanding of prostate cancer therapies. Clinical Science (London, England) 2005;108:1–11. doi: 10.1042/CS20040241. [DOI] [PubMed] [Google Scholar]
- Manning A. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature. 1962;194:252–253. [Google Scholar]
- Maranesi M, Zerani M, Leonardi L, Pistilli A, Arruda-Alencar J, Stabile AM, et al. Gene expression and localization of NGF and its cognate receptors NTRK1 and NGFR in the sex organs of male rabbits. Reproduction in Domestic Animals. 2015;50:918–925. doi: 10.1111/rda.12609. [DOI] [PubMed] [Google Scholar]
- Marques PI, Bernardino R, Fernandes T, NISC Comparative Sequencing Program. Green ED, Hurle B, et al. Birth-and-death of KLK3 and KLK2 in primates: Evolution driven by reproductive biology. Genome Biology and Evolution. 2012;4:1331–1338. doi: 10.1093/gbe/evs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science. 2015;349:1483–1489. doi: 10.1126/science.aab4082. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Science’s STKE. 2004;2004(220):pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Melnik BC, John SM, Schmitz G. Milk: An exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? Journal of Translational Medicine. 2014;12:43. doi: 10.1186/1479-5876-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miah AG, Salma U, Hamano K, Schellander K. Physiological roles of relaxin in prefertilizing activities of spermatozoa. Animal Reproduction Science. 2015;161:1–15. doi: 10.1016/j.anireprosci.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Minami R, Wakabayashi M, Sugimori S, Taniguchi K, Kokuryo A, Imano T, et al. The homeodomain protein defective proventriculus is essential for male accessory gland development to enhance fecundity in Drosophila. PLoS One. 2012;7:e32302. doi: 10.1371/journal.pone.0032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T, Neal DE. The genomic evolution of human prostate cancer. British Journal of Cancer. 2015;113:193–198. doi: 10.1038/bjc.2015.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EH, Innocenti P. Female postmating immune responses, immune system evolution and immunogenic males. Biological Reviews of the Cambridge Philosophical Society. 2012;87:631–638. doi: 10.1111/j.1469-185X.2011.00214.x. [DOI] [PubMed] [Google Scholar]
- Moshitzky P, Fleischmann I, Chaimov N, Saudan P, Klauser S, Kubli E, et al. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Archives of Insect Biochemistry and Physiology. 1996;32:363–374. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<363::AID-ARCH9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Murer V, Spetz JF, Hengst U, Altrogge LM, de Agostini A, Monard D. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3029–3033. doi: 10.1073/pnas.051630698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassis L, Frauman AG, Ohishi M, Zhuo J, Casley DJ, Johnston CI, et al. Localization of angiotensin-converting enzyme in the human prostate: Pathological expression in benign prostatic hyperplasia. The Journal of Pathology. 2001;195:571–579. doi: 10.1002/path.999. [DOI] [PubMed] [Google Scholar]
- Nászai M, Carroll LR, Cordero JB. Intestinal stem cell proliferation and epithelial homeostasis in the adult Drosophila midgut. Insect Biochemistry and Molecular Biology. 2015;67:9–14. doi: 10.1016/j.ibmb.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Omori A, Miyagawa S, Ogino Y, Harada M, Ishii K, Sugimura Y, et al. Essential roles of epithelial bone morphogenetic protein signaling during prostatic development. Endocrinology. 2014;155:2534–2544. doi: 10.1210/en.2013-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampalakis G, Sotiropoulou G. Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochimica et Biophysica Acta. 2007;1776:22–31. doi: 10.1016/j.bbcan.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Panayidou S, Apidianakis Y. Regenerative inflammation: Lessons from Drosophila intestinal epithelium in health and disease. Pathogens. 2013;2:209–231. doi: 10.3390/pathogens2020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Kim BJ, Kang J, Nam TS, Lim JM, Kim HT, et al. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Science Signaling. 2011;4:ra31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- Patel PH, Edgar BA. Tissue design: How Drosophila tumors remodel their neighborhood. Seminars in Cell & Developmental Biology. 2014;28:86–95. doi: 10.1016/j.semcdb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET. Nature Medicine. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Llopis S, Brugarolas J. TFEB, a novel mTORC1 effector implicated in lysosome biogenesis, endocytosis and autophagy. Cell Cycle. 2011;10:3987–3988. doi: 10.4161/cc.10.23.18251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Chen S, Büsser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Current Biology. 2005a;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Peng YC, Joyner AL. Hedgehog signaling in prostate epithelial-mesenchymal growth regulation. Developmental Biology. 2015;400:94–104. doi: 10.1016/j.ydbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Current Biology. 2005b;215:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harbor Perspectives in Biology. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons-Rejraji H, Artonne C, Sion B, Brugnon F, Canis M, Janny L, et al. Prostasomes: Inhibitors of capacitation and modulators of cellular signalling in human sperm. International Journal of Andrology. 2011;34:568–580. doi: 10.1111/j.1365-2605.2010.01116.x. [DOI] [PubMed] [Google Scholar]
- Powers GL, Marker PC. Recent advances in prostate development and links to prostatic diseases. Wiley Interdisciplinary Reviews. Systems Biology and Medicine. 2013;5:243–256. doi: 10.1002/wsbm.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Dominado N, Zoller R, Ng C, Kudyba K, Siddall NA, et al. Ecdysone signaling opposes epidermal growth factor signaling in regulating cyst differentiation in the male gonad of Drosophila melanogaster. Developmental Biology. 2014;394:217–227. doi: 10.1016/j.ydbio.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LT, Pierson RA, et al. The nerve of ovulation-inducing factor in semen. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15042–15047. doi: 10.1073/pnas.1206273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Sirot LK, Wolfner MF. Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18674–18679. doi: 10.1073/pnas.0606228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genetics. 2007;3:e238. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Current Biology. 2014;24:725–730. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. Neural circuitry underlying Drosophila female postmating behavioral responses. Current Biology. 2012;22:1155–1165. doi: 10.1016/j.cub.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Current Biology. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Ronquist G. Prostasomes: Their characterisation: Implications for human reproduction: Prostasomes and human reproduction. Advances in Experimental Medicine and Biology. 2015;868:191–209. doi: 10.1007/978-3-319-18881-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-Nieves AE, González-Reyes A. Genetics and mechanisms of ovarian cancer: Parallels between Drosophila and humans. Seminars in Cell and Developmental Biology. 2014;28:104–109. doi: 10.1016/j.semcdb.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Rudrapatna VA, Cagan RL, Das TK. Drosophila cancer models. Developmental Dynamics. 2012;241:107–118. doi: 10.1002/dvdy.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. The New England Journal of Medicine. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- Rylett CM, Walker MJ, Howell GJ, Shirras AD, Isaac RE. Male accessory glands of Drosophila melanogaster make a secreted angiotensin I-converting enzyme (ANCE), suggesting a role for the peptide-processing enzyme in seminal fluid. The Journal of Experimental Biology. 2007;210:3601–3606. doi: 10.1242/jeb.009035. [DOI] [PubMed] [Google Scholar]
- Schrijvers D. Androgen-independent prostate cancer. Recent Results in Cancer Research. 2007;175:239–249. doi: 10.1007/978-3-540-40901-4_14. [DOI] [PubMed] [Google Scholar]
- Schwedes CC, Carney GE. Ecdysone signaling in adult Drosophila melanogaster. Journal of Insect Physiology. 2012;58:293–302. doi: 10.1016/j.jinsphys.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Schwertfeger KL. Fibroblast growth factors in development and cancer: Insights from the mammary and prostate glands. Current Drug Targets. 2009;10:632–644. doi: 10.2174/138945009788680419. [DOI] [PubMed] [Google Scholar]
- Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, et al. Molecular classification of gastric cancer: A new paradigm. Clinical Cancer Research. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA. TGF-β mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. Journal of Immunology. 2012;189:1024–1035. doi: 10.4049/jimmunol.1200005. [DOI] [PubMed] [Google Scholar]
- Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. The Journal of Clinical Investigation. 2010;120:4478–4492. doi: 10.1172/JCI44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SM, Wolfner MF, Lazzaro BP. Female Drosophila melanogaster suffer reduced defense against infection due to seminal fluid components. Journal of Insect Physiology. 2012;58:1192–1201. doi: 10.1016/j.jinsphys.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Silva M, Niño A, Guerra M, Letelier C, Valderrama XP, Adams GP, et al. Is an ovulation-inducing factor (OIF) present in the seminal plasma of rabbits? Animal Reproduction Science. 2011;127:213–221. doi: 10.1016/j.anireprosci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Simons BW, Hurley PJ, Huang Z, Ross AE, Miller R, Marchionni L, et al. Wnt signaling though beta-catenin is required for prostate lineage specification. Developmental Biology. 2012;371:246–255. doi: 10.1016/j.ydbio.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Findlay GD, Sitnik JL, Frasheri D, Avila FW, Wolfner MF. Molecular characterization and evolution of a gene family encoding both female- and male-specific reproductive proteins in Drosophila. Molecular Biology and Evolution. 2014;31:1554–1567. doi: 10.1093/molbev/msu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, LaFlamme BA, Sitnik JL, Rubinstein CD, Avila FW, Chow CY, et al. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Advances in Genetics. 2009;68:23–56. doi: 10.1016/S0065-2660(09)68002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Wolfner MF, Wigby S. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9922–9926. doi: 10.1073/pnas.1100905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnik JL, Gligorov D, Maeda RK, Karch F, Wolfner MF. Secondary cell expressed genes in the male accessory gland are needed for the female post-mating response in Drosophila melanogaster. Genetics. 2016;202:1029–1041. doi: 10.1534/genetics.115.181644. pii, genetics. 115.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfel MN, Moses KA, Carson JA, Zimmer DB, DeMayo F, Schwartz RJ, et al. Expression of an Nkx3.1-CRE gene using ROSA26 reporter mice. Genesis. 2006;44:550–555. doi: 10.1002/dvg.20250. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Reviews Molecular Cell Biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- Susic-Jung L, Hornbruch-Freitag C, Kuckwa J, Rexer KH, Lammel U, Renkawitz-Pohl R. Multinucleated smooth muscles and mononucleated as well as multinucleated striated muscles develop during establishment of the male reproductive organs of Drosophila melanogaster. Developmental Biology. 2012;2370:86–97. doi: 10.1016/j.ydbio.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Kokuryo A, Imano T, Minami R, Nakagoshi H, Adachi-Yamada T. Isoform-specific functions of Mud/NuMA mediate binucleation of Drosophila male accessory gland cells. BMC Developmental Biology. 2014;14:46. doi: 10.1186/s12861-014-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20697–20702. doi: 10.1073/pnas.1218246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MK, Francis JC, Swain A. The role of Sox9 in prostate development. Differentiation. 2008;76:728–735. doi: 10.1111/j.1432-0436.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Marker PC. Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation. 2006;74:382–392. doi: 10.1111/j.1432-0436.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Tipping M, Perrimon N. Drosophila as a model for context-dependent tumorigenesis. Journal of Cellular Physiology. 2014;229:27–33. doi: 10.1002/jcp.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clinical Cancer Research. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- Torres IL, Rosa-Ferreira C, Munro S. The Arf family G protein Arl1 is required for secretory granule biogenesis in Drosophila. Journal of Cell Science. 2014;127:2151–2160. doi: 10.1242/jcs.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Peyre JB, Asano T, Aigaki T. Visualizing molecular functions and cross-species activity of sex-peptide in Drosophila. Genetics. 2015;200:1161–1169. doi: 10.1534/genetics.115.177550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udby L, Lundwall A, Johnsen AH, Fernlund P, Valtonen-André C, Blom AM, et al. beta-Microseminoprotein binds CRISP-3 in human seminal plasma. Biochemical and Biophysical Research Communications. 2005;333:555–561. doi: 10.1016/j.bbrc.2005.05.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weerden WM, Romijn JC. Use of nude mouse xenograft models in prostate cancer research. Prostate. 2000;43:263–271. doi: 10.1002/1097-0045(20000601)43:4<263::aid-pros5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Veveris-Lowe TL, Kruger SJ, Walsh T, Gardiner RA, Clements JA. Seminal fluid characterization for male fertility and prostate cancer: Kallikrein-related serine proteases and whole proteome approaches. Seminars in Thrombosis and Hemostasis. 2007;33:87–99. doi: 10.1055/s-2006-958467. [DOI] [PubMed] [Google Scholar]
- Vinall RL, Mahaffey CM, Davis RR, Luo Z, Gandour-Edwards R, Ghosh PM, et al. Dual blockade of PKA and NF-kB inhibits H2 relaxin-mediated castrateresistant growth of prostate cancer sublines and induces apoptosis. Hormones & Cancer. 2011;2:224–238. doi: 10.1007/s12672-011-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinänen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nature Genetics. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- Walker SJ, Corrales-Carvajal VM, Ribeiro C. Postmating circuitry modulates salt taste processing to increase reproductive output in Drosophila. Current Biology. 2015;25:2621–2630. doi: 10.1016/j.cub.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- White SJ, Masters ARW, Woolf AS. Renal and bladder epithelial cells. In: Harris A, editor. Epithelial cell culture. Cambridge: Cambridge University Press; 1997. pp. 58–78. [Google Scholar]
- Wigby S, Sirot LK, Linklater JR, Buehner N, Calboli FC, Bretman A, et al. Seminal fluid protein allocation and male reproductive success. Current Biology. 2009;19:751–757. doi: 10.1016/j.cub.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner MF, Partridge L, Lewin S, Kalb JM, Chapman T, Herndon LA. Mating and hormonal triggers regulate accessory gland gene expression in male Drosophila. Journal of Insect Physiology. 1997;43:1117–1123. doi: 10.1016/s0022-1910(97)00062-0. [DOI] [PubMed] [Google Scholar]
- Wu X, Gong S, Roy-Burman P, Lee P, Culig Z. Current mouse and cell models in prostate cancer research. Endocrine-Related Cancer. 2013;20:R155–R170. doi: 10.1530/ERC-12-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier de Carvalho A, Maiato H, Maia AF, Ribeiro SA, Pontes P, Bickmore W, et al. Reed-Sternberg cells form by abscission failure in the presence of functional Aurora B kinase. PLoS One. 2015;10:e0124629. doi: 10.1371/journal.pone.0124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, You C, Chen J. An updated meta-analysis on association between angiotensin I-converting enzyme gene insertion/deletion polymorphism and cancer risk. Tumour Biology. 2014;35:6567–6579. doi: 10.1007/s13277-014-1842-z. [DOI] [PubMed] [Google Scholar]
- Xu B, Hariharan A, Rakshit S, Dressler GR, Wellik DM. The role of Pax2 in mouse prostate development. Prostate. 2012;72:217–224. doi: 10.1002/pros.21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Noll M. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3272–3275. doi: 10.1073/pnas.060018897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Noll M. Dual role of the Pax gene paired in accessory gland development of Drosophila. Development. 2002;129:339–346. doi: 10.1242/dev.129.2.339. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annual Review of Entomology. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, et al. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- Yu S, Cao H, Shen B, Feng J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6:37151–37168. doi: 10.18632/oncotarget.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, et al. Androgen receptor variants occur frequently in castration-resistant prostate cancer metastases. PLoS One. 2011;6:e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cellular Immunology. 2014;292:65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Zielke N, Edgar BA, DePamphilis ML. Endoreplication. Cold Spring Harbor Perspectives in Biology. 2013;5:a012948. doi: 10.1101/cshperspect.a012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]