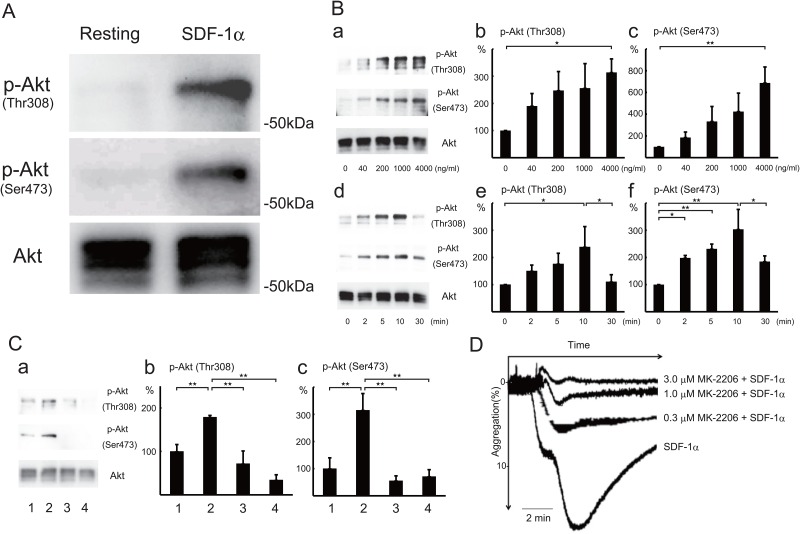
Fig 2. SDF-1α-induced Akt phosphorylation at Thr308 and Ser473 and Akt-dependent platelet aggregation.
(A) Washed human platelets were treated with 500 ng/ml SDF-1α for 10 min at 37°C. Resting platelets (left lane) and SDF-1α-treated platelets (right lane) were lysed in the Laemmli sample buffer containing phosphatase inhibitors and separated by SDS-PAGE. Phosphorylations of Akt at Thr308 (upper panel), Akt at Ser473 (middle panel), and Akt protein (lower panel) were detected by western blotting using specific Akt antibodies. (B) Dose- and time-dependent phosphorylation of Akt in platelets on SDF-1α treatment. Washed human platelets were treated for 10 min at the indicated concentrations (a-c) or at 500 ng/ml SDF-1α for the indicated times (d-f). Cells were lysed in the Laemmli sample buffer containing phosphatase inhibitors and separated by SDS-PAGE. Phosphorylations of Akt at Thr308 and Ser473 were detected by western blotting (a,d). Phosphorylation of Akt at Thr308 (b,e) and Ser473 (c,f) was quantified by densitometry. Data are presented as the mean +/- SD of triplicates. Statistically significant differences (P< .05 shown by *, P< .01 shown by **). (C) Effect of CXCR4 antagonist and PI3 kinase inhibitor on SDF-1α-induced Akt phosphorylation. Western blotting (a) and measurement of Akt phosphorylation at Thr308 (b) and Ser473 (c) in resting platelets (lane 1), 500 ng/ml SDF-1α-treated platelets (lane 2), and 500 ng/ml SDF-1α-treated platelets with pretreatment with 5 μg/ml AMD3100 (lane 3), or 30 μM LY294002 (lane 4) for 10 min. Data are presented as the mean +/- SD of triplicates. **Statistically significant differences (P< .01). (D) Inhibition of SDF-1α-induced platelet aggregation by Akt inhibitor. SDF-1α(200 ng/ml)-induced platelet aggregation without or with pretreatment with 0.3, 1, or 3 μM MK-2206, an Akt inhibitor, for 10 min (2 donors, n = 3: a single measurement from one donor and a duplicate measurement from another donor on the same day). Bar represents 2 min.