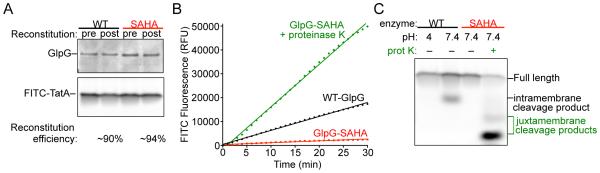
Figure 4.
Key parameters for inducible intramembrane protease assay development. (A) Representative samples taken pre- and post-reconstitution for reactions with either wildtype GlpG or its catalytic mutant (SAHA is GlpG-S201A+H254A) were resolved electrophoretically and imaged for GlpG protease levels by Krypton infrared protein staining followed by Odyssey infrared scanning (upper panel) and for FITC-TatA using a Typhoon fluorescence scanner (lower panel). Reconstitution efficiency of 90-95% was observed for both the protease and substrate. (B) Real-time reaction time courses show a linear increase in FITC-fluorescence over time for wildtype GlpG (black line) compared to a negligible but common ‘drift’ in fluorescence with the catalytic mutant (red line). A robust fluorescence signal is generated when proteinase K is added to the SAHA reaction (green line). (C) SDS gel electrophoresis followed by fluorescent imaging of reaction products confirms that the FITC fluorescence signal detected for wildtype GlpG in real-time corresponds to an intramembrane proteolytic cleavage product (lane 2) that is not detected when the catalytic residues are mutated (lane 3) or when the wildtype enzyme is assayed at pH 4 (lane 1). Smaller reaction products corresponding to cleavage sites outside the membrane were detected in the presence of proteinase K (lane 4).